Chapter: Introduction to Human Nutrition: The Vitamins
Vitamin K - Human Nutrition
Vitamin K
Vitamin K was discovered as a result of investigations into the cause of a bleeding disorder (hemorrhagic disease) of cattle fed on silage made from sweet clover and of chickens fed on a fat-free diet. The missing factor in the diet of the chickens was identified as vitamin K, whereas the problem in the cattle was that the feed contained dicumarol, an antagonist of the vitamin.
Since the effect of an excessive intake of dicumarol was severely impaired blood clotting, it was isolated and tested in low doses as an anticoagulant, for use in patients at risk of thrombosis. Although it was effec-tive, it had unwanted side-effects, and synthetic vitamin K antagonists were developed for clinical use as anticoagulants. The most commonly used of these is warfarin, which is also used, in larger amounts, to kill rodents.
Vitamers
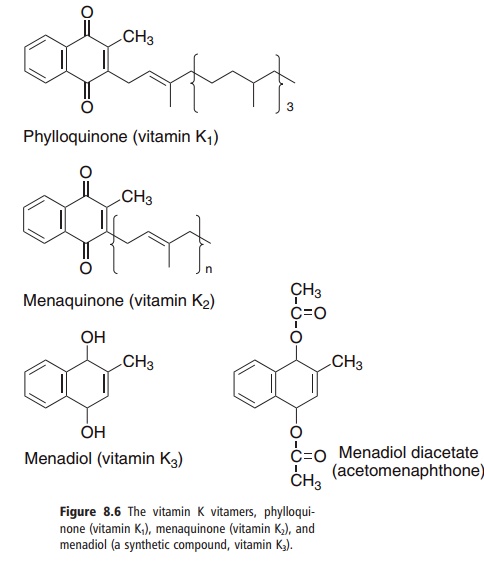
Three compounds have the biological activity of vitamin K (Figure 8.6):
● phylloquinone, the normal dietary source, found in green leafy vegetables;
● menaquinones, a family of related compounds syn-thesized by intestinal bacteria, with differing lengths of the side-chain;
● menadiol and menadiol diacetate, synthetic com-pounds that can be metabolized to phylloquinone.
Dietary sources, bacterial synthesis and metabolism
Phylloquinone has a role in photosynthesis, and therefore it is found in all green leafy vegetables; the richest sources are spring (collard) greens, spinach, and Brussels sprouts. In addition, soybean, rapeseed, cottonseed, and olive oils are relatively rich in vitamin K, although other oils are not.
About 80% of dietary phylloquinone is normally absorbed into the lymphatic system in chylomicrons, and is then taken up by the liver from chylomicron remnants and released into the circulation in VLDLs.
Intestinal bacteria synthesize a variety of menaqui-nones, which are absorbed to a limited extent from the large intestine, again into the lymphatic system, cleared by the liver, and released in VLDLs. It is often suggested that about half of the requirement for vitamin K is met by intestinal bacterial synthesis, but there is little evidence for this, other than the fact that about half of the vitamin K in liver is phylloquinone and the remainder a variety of menaquinones. It is not clear to what extent the menaquinones are bio-logically active. It is possible to induce signs of vitamin K deficiency simply by feeding a phylloquinone-defi-cient diet, without inhibiting intestinal bacterial action.
The synthetic compound menadiol is absorbed largely into the hepatic portal system, and undergoes alkylation in the liver to yield menaquinone-4, which is released together with phylloquinone and other menaquinones in VLDLs.
Metabolic functions of vitamin K
Although it has been known since the 1920s that vitamin K was required for blood clotting, it was not until the 1970s that its precise function was estab-lished. It is the cofactor for the carboxylation of glutamate residues in the postsynthetic modification of proteins to form the unusual amino acid γ-carboxyglutamate, abbreviated to Gla (Figure 8.7).
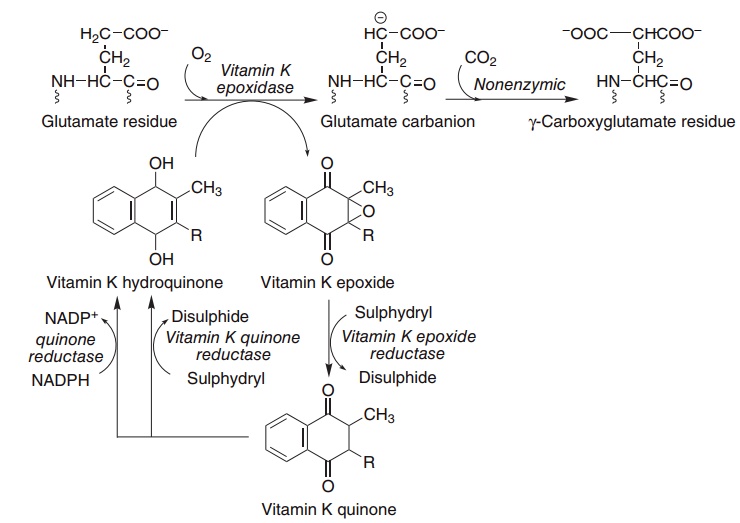
Figure 8.7 Role of vitamin K in the carboxylation of glutamate. Vitamin K epoxidase (EC 1.14.99.20), warfarin-sensitive epoxide/quinone reduc-tase (EC 1.1.4.1), warfarin-insensitive quinone reductase (EC 1.1.4.2).
In the presence of warfarin, vitamin K epoxide cannot be reduced back to the active hydroquinone, but accumulates and is excreted as a variety of conju-gates. However, if enough vitamin K is provided in the diet, the quinone can be reduced to the active hydroquinone by the warfarin-insensitive enzyme, and carboxylation can continue, with stoichiometric utilization of vitamin K and excretion of the epoxide. High doses of vitamin K are used to treat patients who have received an overdose of warfarin, and at least part of the resistance of some populations of rats to the action of warfarin is due to a high consumption of vitamin K from maram grass, although there are also genetically resistant populations of rodents.
Prothrombin and several other proteins of the blood clotting system (factors VII, IX and X, and proteins C and S) each contain between four and six γ-carboxyglutamate residues per mole. γ-Carboxy glutamate chelates calcium ions, and so permits the binding of the blood clotting proteins to lipid mem-branes. In vitamin K deficiency, or in the presence of an antagonist such as warfarin, an abnormal precur-sor of prothrombin (preprothrombin) containing little or no γ-carboxyglutamate is released into the circulation. Preprothrombin cannot chelate calcium or bind to phospholipid membranes, and so is unable to initiate blood clotting. Preprothrombin is some-times known as PIVKA: the protein induced by vitamin K absence.
Other vitamin K-dependent proteins
It has long been known that treatment of pregnant women with warfarin or other anticoagulants can lead to bone abnormalities in the child: the fetal war-farin syndrome. Two proteins in bone matrix contain γ-carboxyglutamate: osteocalcin and a less well char- acterized protein simply known as bone matrix Gla protein. Osteocalcin is interesting in that as well as γ-carboxyglutamate, it also contains hydroxyproline, so its synthesis is dependent on both vitamins K and C; in addition, its synthesis is induced by vitamin D, and the release into the circulation of osteocalcin provides a sensitive index of vitamin D action. It constitutes some 1–2% of total bone protein, and modifies the crystallization of bone mineral. The matrix Gla protein is found in a variety of tissues, and acts to prevent mineralization of soft connective tissue.
The fetal warfarin syndrome involves neurological as well as bone abnormalities. The vitamin K-dependent carboxylase is expressed in different brain regions at different times during embryological devel-opment, and the product of the growth arrest-specific gene 6 (Gas6) is a Gla-containing growth factor that is important in the regulation of growth and development, and the regulation of apoptosis and cell survival.
Vitamin K deficiency and requirements
Apart from deliberate experimental manipulation, vitamin K deficiency is unknown, and determination of requirements is complicated by a lack of informa-tion on the importance of menaquinones synthesized by intestinal bacteria.
The classical way of determining vitamin K status, and monitoring the efficacy of anticoagulant therapy, is by measuring the time required for the formation of a fibrin clot in citrated blood plasma after the addi-tion of calcium ions and thromboplastin: the pro-thrombin time. A more sensitive index is provided by direct measurement of preprothrombin in plasma, most commonly by immunoassay using antisera against preprothrombin that do not react with prothrombin.
Based on determination of clotting time, and direct measurement of prothrombin and preprothrombin, an intake of 1 μg/kg body weight per day is consid-ered adequate; this forms the basis of reference intakes of between 65 and 80 μg/day for adults.
A small number of newborn infants have very low reserves of vitamin K and are at risk of potentially fatal hemorrhagic disease. It is therefore generally rec-ommended that all neonates should be given a single prophylactic dose of vitamin K.
Toxicity and drug interactions
There is no evidence that phylloquinone has any sig-nificant toxicity. However, high intakes can overcome the effects of warfarin and other anticoagulants. This means that patients who are being treated with war-farin could overcome the beneficial effects of their medication if they took supplements of vitamin K. The danger is that if their dose of warfarin is increased to counteract the effects of the vitamin supplements and they then stop taking the supplements, they would be receiving considerably too much warfarin and would be at risk of hemorrhage.
It is unlikely that a normal diet could provide a sufficient excess of vitamin K to lead to problems, but habitual consumption of especially rich sources could result in intakes close to those that antagonize thera-peutic warfarin. A diet containing relatively large amounts of foods prepared with vitamin K-rich oils may pose a risk.
Related Topics