Chapter: Introduction to Human Nutrition: The Vitamins
Vitamin B1 (thiamin)
Vitamin B1 (thiamin)
Historically, thiamin deficiency affecting the peripheral nervous system (beriberi) was a major public health problem in south-east Asia following the intro-duction of the steam-powered mill that made highly polished (and therefore thiamin-depleted) rice widely available. There are still sporadic outbreaks of defi-ciency among people whose diet is rich in carbohy-drate and poor in thiamin. More commonly, thiamin deficiency affecting the heart and central nervous system is a problem in people with an excessive con-sumption of alcohol, to the extent that there was a serious suggestion in Australia at one time that thiamin should be added to beer.
The structures of thiamin and the coenzyme thiamin diphosphate are shown in Figure 8.8.
Thiamin is widely distributed in foods, with pork being an especially rich source; potatoes, whole-grain cereals, meat, and fish are the major sources in most diets. Like other water-soluble vitamins, thiamin is readily lost by leaching into cooking water; further-more, it is unstable to light, and although bread and flour contain significant amounts of thiamin, much of this can be lost when baked goods are exposed to sunlight in a shop window.
Thiamin is also destroyed by sulfites, and in potato products that have been blanched by immersion in sulfite solution there is little or no thiamin remaining. Polyphenols, including tannic acid in tea and betel nuts, also destroy thiamin, and have been associated with thiamin deficiency.
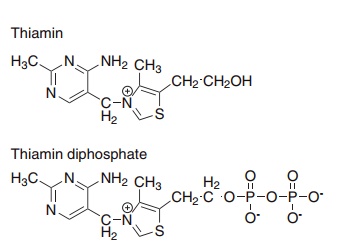
Figure 8.8 Thiamin (vitamin B1) and the coenzyme thiamin diphosphate.
Thiaminases that catalyze base exchange or hydrolysis of thiamin are found in microorganisms (including some that colonize the gut), a variety of plants, and raw fish. The presence of thiaminase in fermented fish is believed to be a significant factor in the etiology of thiamin deficiency in parts of south-east Asia.
Absorption and metabolism of thiamin
Thiamin is absorbed in the duodenum and proximal jejunum, and then transferred to the portal circula-tion by an active transport process that is inhibited by alcohol. This may explain why alcoholics are espe-cially susceptible to thiamin deficiency.
Tissues take up both free thiamin and thiamin monophosphate, then phosphorylate them further to yield thiamin diphosphate (the active coenzyme) and, in the nervous system, thiamin triphosphate.
Some free thiamin is excreted in the urine, increas-ing with diuresis, and a significant amount may also be lost in sweat. Most urinary excretion is as thio-chrome, the result of non-enzymic cyclization, as well as a variety of products of side-chain oxidation and ring cleavage.
There is little storage of thiamin in the body, and biochemical signs of deficiency can be observed within a few days of initiating a thiamin-free diet.
Metabolic functions of thiamin
Thiamin has a central role in energy-yielding metabo-lism, and especially the metabolism of carbohydrates. Thiamin diphosphate (also known as thiamin pyro-phosphate, see Figure 8.8) is the coenzyme for three oxidative decarboxylation reactions: pyruvate dehy-drogenase in carbohydrate metabolism, α-keto-glutarate dehydrogenase in the citric acid cycle, and the branched-chain keto-acid dehydrogenase involved in the metabolism of leucine, isoleucine, and valine. These three enzymes are multienzyme complexes that catalyze oxidative decarboxylation of the substrate linked to reduction of enzyme-bound lipoamide, and eventually reduction of NAD+ to NADH.
Thiamin diphosphate is also the coenzyme for transketolase, in the pentose phosphate pathway of carbohydrate metabolism. This is the major pathway of carbohydrate metabolism in some tissues, and an important alternative to glycolysis in all tissues, being the source of half of the NADPH required for fatty acid synthesis.
Thiamin triphosphate has a role in nerve conduc-tion, as the phosphate donor for phosphorylation of a nerve membrane sodium transport protein.
Thiamin deficiency
Thiamin deficiency can result in three distinct syndromes:
● a chronic peripheral neuritis, beriberi, which may or may not be associated with heart failure and edema
● acute pernicious (fulminating) beriberi (shoshin beriberi), in which heart failure and metabolic abnormalities predominate, with little evidence of peripheral neuritis
● Wernicke’s encephalopathy with Korsakoff’s psy-chosis, a thiamin-responsive condition associated especially with alcohol and narcotic abuse.
In general, a relatively acute deficiency is involved in the central nervous system lesions of the Wernicke– Korsakoff syndrome, and a high energy intake, as in alcoholics, is also a predisposing factor. Dry beriberi is associated with a more prolonged, and presumably less severe, deficiency, and a generally low food intake, whereas higher carbohydrate intake and physical activity predispose to wet beriberi.
Dry beriberi
Chronic deficiency of thiamin, especially associated with a high carbohydrate diet, results in beriberi, which is a symmetrical ascending peripheral neuritis. Initially, the patient complains of weakness, stiffness and cramps in the legs, and is unable to walk more than a short distance. There may be numbness of the dorsum of the feet and ankles, and vibration sense may be diminished. As the disease progresses, the ankle jerk reflex is lost, and the muscular weakness spreads upwards, involving first the extensor muscles of the foot, then the muscles of the calf, and finally the extensors and flexors of the thigh. At this stage there is pronounced toe and foot drop: the patient is unable to keep either the toe or the whole foot extended off the ground. When the arms are affected there is a similar inability to keep the hand extended: wrist drop.
Wet beriberi
The heart may also be affected in beriberi, with dilata-tion of arterioles, rapid blood flow, and increased pulse rate leading to right-sided heart failure and edema, so-called wet beriberi. The signs of chronic heart failure may be seen without peripheral neuritis. The arteriolar dilatation probably results from high circulating concentrations of lactate and pyruvate as a result of impaired activity of pyruvate dehydrogenase.
Acute pernicious (fulminating) beriberi: shoshin beriberi
Heart failure without increased cardiac output, and no peripheral edema, may also occur acutely, associ-ated with severe lactic acidosis. This was a common presentation of deficiency in Japan, where it was called shoshin (meaning acute) beriberi; in the 1920s some 26 000 deaths a year were recorded.
With improved knowledge of the cause and improved nutritional status, the disease has become more or less unknown, although in the 1980s it reappeared among Japanese adolescents consuming a diet based largely on such high-carbohydrate, low-nutrient, foods as sweet carbonated drinks, “instant” noodles, and polished rice. It also occurs among alcoholics, when the lactic acidosis may be life-threatening, without clear signs of heart failure. Acute beriberi has also been reported when previously starved subjects are given intravenous glucose.
Wernicke–Korsakoff syndrome
Whereas peripheral neuritis, acute cardiac beriberi and lactic acidosis occur in thiamin deficiency associ- ated with alcohol misuse, the more usual presentation is as the Wernicke–Korsakoff syndrome, due to central nervous system lesions.
Initially, there is a confused state, Korsakoff’s psy-chosis, which is characterized by confabulation and loss of recent memory, although memory for past events may be unimpaired. Later, clear neurological signs develop: Wernicke’s encephalopathy. This is characterized by nystagmus and extraocular palsy. Post-mortem examination shows characteristic brain lesions.
Like shoshin beriberi, Wernicke’s encephalopathy can develop acutely, without the more gradual development of Korsakoff’s psychosis, among previously starved patients given intravenous glu-cose and seriously ill patients given parenteral hyperalimentation.
Thiamin requirements
Because thiamin has a central role in energy-yielding, and especially carbohydrate, metabolism, require-ments depend mainly on carbohydrate intake, and have been related to “non-fat calories.” In practice, requirements and reference intakes are calculated on the basis of total energy intake, assuming that the average diet provides 40% of energy from fat. For diets that are lower in fat, and hence higher in carbo-hydrate, thiamin requirements may be somewhat higher.
From depletion/repletion studies, an intake of at least 0.2 mg of thiamin/1000 kcal is required to prevent the development of deficiency signs and maintain normal urinary excretion, but an intake of 0.23 mg/1000 kcal is required for a normal transketo-lase activation coefficient .
Reference intakes are calculated on the basis of 100 μg/MJ (0.5 mg/1000 kcal) for adults consuming more than 2000 kcal/day, with a minimum require-ment for people with a low energy intake of 0.8– 1.0 mg/day to allow for metabolism of endogenous substrates.
Assessment of thiamin status
The impairment of pyruvate dehydrogenase in thiamin deficiency results in a considerable increase in the plasma concentrations of lactate and pyruvate. This has been exploited as a means of assessing thiamin status, by measuring changes in the plasma concentrations of lactate and pyruvate after an oral dose of glucose and mild exercise. The test is not specific for thiamin deficiency since a variety of other conditions can also result in metabolic acidosis. Although it may be useful in depletion/repletion studies, it is little used nowadays in assessment of nutritional status.
Whole blood total thiamin below 150 nmol/l is considered to indicate deficiency. However, the changes observed in depletion studies are small. Even in patients with frank beriberi the total thiamin con-centration in erythrocytes is only 20% lower than normal, so whole blood thiamin is not a sensitive index of status.
Although there are several urinary metabolites of thiamin, a significant proportion is excreted either unchanged or as thiochrome, and therefore the urinary excretion of the vitamin (measured as thio-chrome) can provide information on nutritional status. Excretion decreases proportionally with intake in adequately nourished subjects, but at low intakes there is a threshold below which further reduction in intake has little effect on excretion.
The activation of apo-transketolase in erythrocyte lysate by thiamin diphosphate added in vitro has become the most widely used and accepted index of thiamin nutritional status. Apo-transketolase is unstable both in vivo and in vitro, so problems may arise in the interpretation of results, especially if samples have been stored for any appreciable time. An activation coefficient >1.25 is indicative of deficiency, and <1.15 is considered to reflect adequate thiamin status.
Related Topics