Chapter: Psychology: Learning
Varieties of Learning
VARIETIES OF LEARNING
Overall, the attempt to find
general principles of learning—principles that apply to virtually all
species—has been rather successful. This is why we can gain insights into human
depression by studying helplessness in dogs, and it’s how we’ve increased our
understanding of human drug addiction—thanks to research on clas-sical
conditioning in rats. Other examples of learning phenomena shared across
species are easy to find.
We need to acknowledge, though,
that there are also important differences from one species to the next in how
learning proceeds. These differences are often best understood by taking a
biological perspective on learning—a perspective that highlights the actual function of learning in each species’
natural environment (see, for example, Bolles &Beecher, 1988; Domjan, 2005;
Rozin & Schull, 1988).
Biological Influences on Learning: Belongingness
In the early days of learning
theory, investigators widely believed that animals (both humans and others) are
capable of connecting any CS to any US in classical condition-ing, and of
associating virtually any response with any reinforcer in instrumental
con-ditioning. A child could be taught that a tone signaled the approach of
dinner or that a flashing light or a particular word did. Likewise, a rat could
be trained to press a lever to get food, water, or access to a sexually
receptive mate.
But a great deal of evidence speaks against this idea; instead, each species seems pre-disposed to form some associations and not others. The predispositions put biologicalconstraints on that species’ learning, governing what the species can learn easily andwhat it can learn only with difficulty. These associative predispositions are probably hardwired and likely to be a direct product of our evolutionary past (Rozin & Kalat, 1971, 1972; Seligman & Hager, 1972).
TASTE AVERSION LEARNING
A central example of the
biological constraints on learning comes from studies of tasteaversion learning. These studies make it clear that, from the
organism’s viewpoint, somestimuli belong together and some do not (Domjan,
1983, 2005; Garcia & Koelling, 1966).
To understand this phenomenon, we
need to begin with the fact that when a wild rat encounters a novel food, it
generally takes only a small bite at first. If the rat suffers no ill effects
from this first taste, it will return (perhaps a day or two later) for a second
helping and will gradually make the food a part of its regular diet. But what
if this novel food is harmful, either because of some natural toxin or an
exterminator’s poison? In that case, the initial taste will make the rat sick;
but because it ate only a little of the food, the rat will probably recover.
Based on this experience, though, the rat is likely to develop a strong
aversion to that particular flavor, so it never returns for a second dose of
the poison.
This sort of learning is easily
documented in the laboratory. The subjects, usually rats, are presented with a
food or drink that has a novel flavor—perhaps water with some vanilla added.
After drinking this flavored water, the rats are exposed to X-ray radiation—not
enough to injure them, but enough to make them ill. After they recover, the
rats show a strong aversion to the taste of vanilla and refuse to drink water
flavored in this way (Figure 7.30).
This learned taste aversion is
actually based on classical conditioning. The flavor (here, vanilla) serves as
the CS, and the sensation of being sick serves as the US. This is, however, a
specialized type of classical conditioning that is distinct from other forms in
the sheer speed of learning: One pairing of a taste + illness is all it takes
to establish the connection between them. This one-trial learning is obviously much faster than the speed of
ordinary classical conditioning. What’s more, this form of condi-tioning is
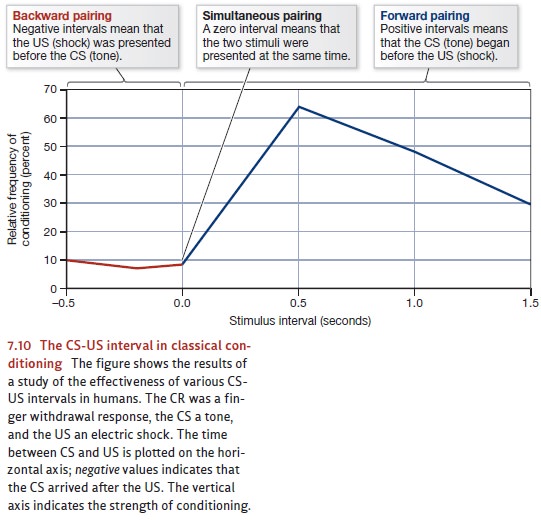
distinctive in its timing requirements. In most classical conditioning, the CS
must be soon followed by the US; if too much time passes between these two
stim-uli, the likelihood of conditioning is much reduced (see Figure 7.10). In
taste aversion learning, in contrast, conditioning can be observed even if several
hours elapse between the CS and the US.
Learned taste aversions are also
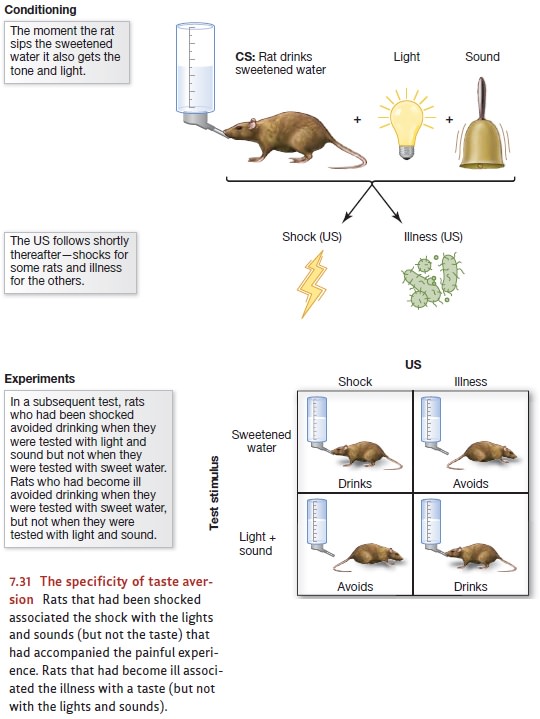
remarkable for their specificity. In one early study, thirsty rats were allowed
to drink sweetened water through a tube. Whenever the rats licked the nozzle of
this tube, a bright light flashed and a loud clicking noise sounded. Thus, the
sweetness, bright light, and loud noise were always grouped together; if one
was presented, all were presented. One group of these rats then received an
electric shock to the feet. A second group was exposed to a dose of X-rays
strong enough to cause illness.
Notice, then, that we have two
different USs—illness for one group and foot shock for the other. Both groups
also have received a three-part CS: sweet + bright + noisy. The question is:
How will the animals put these pieces together—what will get associ-ated with
what?
To find out, the experimenters
tested the rats in a new situation. They gave some of the rats sweetened water,
unaccompanied by either light or noise. Rats that had received foot shock
showed no inclination to avoid this water; apparently, they didn’t associate
foot shock with the sweet flavor. However, rats that had been made ill with
X-rays refused to drink this sweetened water; they associated their illness
with the taste (Figure 7.31).
Another group of rats were tested
with unflavored water accompanied by the light and sound cues that were present
during training. Now the pattern was reversed. Rats that had become ill showed
no objection to this water. For them, the objectionable (sweet) taste was
absent from this test stimulus, and they didn’t associate their illness with
the sights and sounds that were present during the test. However, rats that had
been shocked earlier refused to drink this water; in their minds, pain was associated
with bright lights and loud clicks (Garcia & Koelling, 1966).
For the rat, therefore, taste
goes with illness, and sights and sounds go with exter-nally induced pain. And
for this species, this pattern makes biological sense. Illness in wild rats is
likely to have been caused by harmful or tainted food, and rats generally
select their food largely on the basis of flavor. So there’s survival value in
the rats being able to learn quickly about the connection between a particular
flavor and illness; this will provide useful information for them as they
select their next meal, ensuring that they don’t resample the harmful berries
or poisoned meat.
Using this logic, one might
expect species that choose foods on the basis of other attributes to make
different associations. For example, many birds make their food choices from a
distance, relying on the food’s visual appearance. How will this behavior
affect the data? In one study, quail were given blue, sour water to drink and
were then given a low dose of poison—enough to make them ill, but not enough to
harm them. Some of the birds were later tested with blue, unflavored water;
others were tested with water that was sour but colorless. The results showed
that the quail had developed a strong aversion to blue water but no aversion to
the sour water. They learned which water was safe based on its color rather
than its taste (Wilcoxin, Dragoin, & Kral, 1971).
Clearly, what belongs with what
depends upon the species. Birds are predisposed to associate illness with
visual cues. Rats (and many other mammals) associate illness with taste. In
each case, the bias makes the animal more prepared to form certain
asso-ciations and far less prepared to form others (Seligman, 1970).

We should also mention that taste
aversion learning, as important as it is, is just one example of prepared learning (Figure 7.32). We
mentioned a different example in the Prologue: Humans in one experiment were
shown specific pictures as the CS and received electric shocks as the US. When
the pictures showed flowers or mushrooms, learning was relatively slow. When the
pictures showed snakes, learning was much quicker. The impli-cation is that
humans (and many other primates) are innately prepared to associate the sight
of a snake with unpleasant or even painful experiences (Ă–hman & Mineka,
2003; Ă–hman & Soares, 1993; also Domjan, Cusato, & Krause, 2004).
These results may help us
understand why so many people are afraid of snakes and why strong phobias for
snakes are relatively common. Perhaps it’s not surprising that many cultures
regard snakes as the embodiments of evil. All these facts may simply be the
result of prepared learning in our species—our innate tendencies toward making
certain associations but not others.
BIOLOGICAL CONSTRAINTS ON INSTRUMENTAL CONDITIONING
Prepared learning can also be
demonstrated in instrumental conditioning because, from an animal’s viewpoint,
certain responses belong with some rewards and not oth-ers (Shettleworth,
1972). For example, pigeons can easily be taught to peck a lit key to obtain
food or water, but it’s extremely difficult to train a pigeon to peck in order
to escape electric shock (Hineline & Rachlin, 1969). In contrast, pigeons
can easily be taught to hop or flap their wings to get away from shock, but
it’s difficult to train the pigeon to produce these same responses in order to
gain food or water.
Once again, this pattern makes
good biological sense. The pigeon’s normal reaction to danger is to hop away or
break into flight, so the pigeon is biologically prepared to associate these
responses with aversive stimuli such as electrical shock. Pecking, in
con-trast, is not part of the pigeon’s innate defense pattern, so it’s
difficult for the pigeon to learn pecking as an escape response (Bolles, 1970).
Conversely, since pecking is what pigeons do naturally when they eat, the pigeon
is biologically prepared to associate this response with food or drink; it’s no
wonder, then, that pigeons easily learn to make this association in the
psychologist’s laboratory.
Different Types of Learning
It seems therefore that we need
to “tune” the laws of learning on a case-by-case basis to accommodate the fact
that a given species learns some relationships easily, others only with
difficulty, and still others not at all. This tuning builds some flexibility
into the laws of learning; but it allows us to retain the idea that there are general laws, appli-cable (with the
appropriate tuning) to all species and to all situations. Other evidence
suggests, though, that we must go further than this, because some types of
learning follow their own specialized rules and depend on specialized
capacities found in that species and few others. On this basis, we need to do
more than adjust the laws of learn-ing. We may also need some entirely new
laws—laws that are specific to the species that does the learning and to what’s
being learned (Gallistel, 1990; Roper, 1983).
As one example, consider the
Clark’s nutcracker, a bird that makes its home in the American Southwest
(Figure 7.33). In the summer, this bird buries thousands of pine nuts in
various hiding places over an area of several square miles. Then, throughout
the winter and early spring, the nutcracker flies back again and again to dig
up its thou-sands of caches. The bird doesn’t mark its cache sites in any
special way. Instead, it relies on memory to find its stash—a remarkable feat
that few of us could duplicate.

The Clark’s nutcracker has
various anatomical features that support its food-hoarding activities—for
example, there’s a special pouch under its tongue that it fills with pine nuts
when flying to find a hiding place. The bird’s extraordinary ability to learn a
huge number of geographical locations, and then to remember these locations for
the next few months, is probably a similar evolutionary adaptation. Like the
tongue pouch, this learning ability is a specialty of this species: Related
birds like jays and crows don’t store food in this way; and, when tested, they
have a correspondingly poorer spa-tial memory (D. Olson, 1991; Shettleworth,
1983, 1984, 1990).
Many phenomena of animal learning—in
birds, fish, and mammals—reveal similar specializations. In each case, the
organism has some extraordinary ability not shared even by closely related
species. In each case, the ability has obvious survival value and seems quite
narrow. The Clark’s nutcracker, for example, has no special skill in
remem-bering pictures or shapes; instead, its remarkable memory comes into play
only in the appropriate setting—when hiding and then relocating buried pine
nuts. Similarly, many birds show remarkable talent in learning the particular
songs used by their species. This skill, however, can be used for no other
purpose: A zebra finch easily mas-ters the notes of the zebra finch’s song but
is utterly inept at learning any other (non-musical) sequence of similar length
and complexity. Truly, then, these are specialized learning abilities—only one
or a few species have them, and they apply only to a partic-ular task crucial
for their members’ survival (Gallistel, 1990; Marler, 1970).
But what about humans? We’ve
emphasized that a great deal of human behavior—just like the behavior of every
animal species—is governed by principles of habituation as well as classical
and operant conditioning. But, even so, some of our behavior is the product of
distinctly human forms of learning. One exam-ple involves the processes through
which humans learn language. These processes seem controlled by innate
mechanisms that guide the learning and make it possible for us to achieve
remarkable linguistic competence by the time we’re 3 years old. Humans also
have remarkable inferen-tial abilities that allow us to gain broad sets of new
beliefs, based on events we’ve observed or information we’ve received from
others; and these new beliefs can pro-foundly affect our behavior.
Similarities in How Different Species Learn
In short, there are certainly
differences—as well as crucial similarities—in how species learn, and, as we’ve
noted, the differences make good biological sense. After all, each species
lives in its own distinctive environment and needs its own set of skills, and
so it may need to learn in its own ways. But what about the similarities? After
all, the rats and pigeons we study in the laboratory don’t gather food the way
a human does. They don’t communicate with their fellows the way a human does.
Their nervous systems are much simpler than ours. It wouldn’t be surprising,
therefore, if they learned in different ways than we do. Yet, as we’ve
repeatedly noted, the major phenomena of both classical and instrumental
conditioning apply across species— whether we’re considering humans, rats,
pigeons, cats, dogs, fish, or even some types of snails (Couvillon &
Bitterman, 1980; E. Kandel, 2007).
How should we think about this
point? Why do such diverse creatures share certain types of learning? The
answer lies in the fact that all of these creatures, no matter what their
evolutionary history or ecological niche, share certain needs. For example,
virtu-ally all creatures are better off if they can prepare themselves for
upcoming events, and to do this they need some way of anticipating what will
happen to them in the near future. It’s no wonder, then, that many species have
developed nervous systems that support classical conditioning.
Similarly, in the world we all
inhabit, important outcomes are often influenced by one’s behavior, so it pays
for all species to repeat actions that have worked well in the past and to
abandon actions that haven’t succeeded. Hence we might expect natural selection
to have favored organisms capable of learning about the consequences of their
actions and able to adjust their future behavior accordingly. In other words,
we’d expect natural selection to have favored organisms capable of instrumental
conditioning.
Of course, people are different
from pigeons, and pigeons from sea slugs; no one is questioning these points.
Even so, it seems that there are some types of learning that all species need
to do.
Related Topics