Chapter: Genetics and Molecular Biology: Lambda Phage Integration and Excision
Use of Transducing Phage to Study Integration and Excision
Use of Transducing Phage to Study Integration
and Excision
One use of transducing phage is to
demonstrate that lambda normally integrates and excises at precisely the same
point. Of course, lambda can be integrated and excised many times from the
bacterial att region, and the region
apparently suffers no harm. Nonetheless, how do we know, without sequencing,
that bases are not inserted or deleted in the process? The integration of
lambda into secondary att sites
provided the proof that, as far as the sequence of the host chromosome is
concerned, excision is the exact opposite of integration.
When
lambda integrates into a secondary att
site, the gene into which lambda has inserted is disrupted and therefore
inactivated, but when the lambda is induced and excises from these sites, the
majority of the surviving cells possess a perfectly normal gene. Few of the
lambda improperly excise and produce transducing phage as described in the
previous section. That is, except for the products of the rare improper
excision events, no nucleotides are inserted or deleted at the pseudo att site. Therefore it is reasonable to
infer that the integration and excision cycle at the normal att site similarly does not alter its
sequence. For example, one site for secondary lambda integration is a gene
coding for a protein required for proline synthesis. The insertion of lambda
makes the cells Pro-, but heat-pulse curing leaves the cells Pro+
(Fig. 18.7).
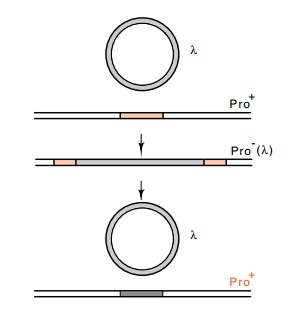
Figure
18.7 Integration oflambda phage into a
pseudo att site in a gene inactivates
that gene, but excision restores the original nucleotide sequence and the gene
is reactivated.
A second
use of transducing phage is in the study of the biochemistry of the integration
and excision reactions themselves. These site-specific recombination events
take place at the att regions, but
the partners need not be confined to a phage and host chromosome. For example,
the enzymatic equivalent of an excision reaction can be performed between λdgaland a λpbioto form a λ and a λdgal-bio. To
detect these recombi-nation products, the input phage must be genetically
marked. This can be done by using nonsense mutations located in the A and R genes, which are at opposite ends of the lambda DNA. The cross
might therefore be between λdgalA-R+ and λpbioA+R-; the
frequency of genera-tion of wild-type lambda, those able to form plaques on su-
cells, could
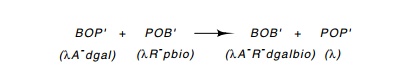
be measured. Crosses between all combinations of att regions can be performed by similar
approaches. The main results of such studies examining the site-specific
recombination events catalyzed by Int and Xis proteins are that all
combinations of att regions will
recombine, but at different rates, and that the Xis protein is required only
for the excision type of reaction.
Related Topics