Chapter: Genetics and Molecular Biology: Lambda Phage Integration and Excision
The Double att Phage, att.squ
The Double att
Phage, att2
The preceding sections have described a little of
what has been learned from genetic experiments about lambda’s ability to
integrate and excise. One way to study the biochemistry of integration and
excision is to construct an in vitro
system that mimics the in vivo
reaction. The first requirement for integration or excision is to bring the two
participating molecules close together. In
vivo, this requirement is partially met as a simple consequence of the fact
that both DNA molecules are confined to the volume of the cell and therefore
are held in close proximity. The in vitro
integration or excision reaction should be greatly speeded if thetwo att regions similarly can be forced
close to one another. One way to accomplish this is to place both att sites on the same DNA molecule. Then
the concentration of one att site in
the vicinity of the other is, of necessity, high.
This section describes the isolation and properties
of such a double att phage, and the
following sections describe its use and the use of asimilar phage to study the
integration and excision reactions more deeply both in vivo and in vitro.
Some distance to either side of an integrated
lambda are two sites that the host recombination system will recombine at a
reasonable frequency. This event excises a phage that has picked up host DNA
from both sides of the phage integration site (Fig. 18.8). Ordinarily this
event cannot be detected because the resulting DNA is too long to be packaged
in a lambda coat, but if several deletions have first been put into lambda,
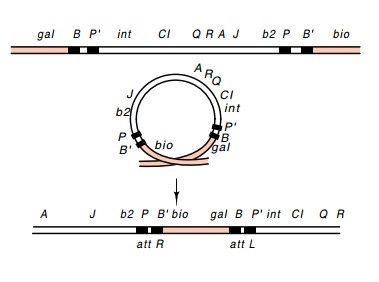
Figure
18.8 Recombinationbetween two points
beyond the ends of an integrated lambda phage generated the λatt2phage containingattRand
attL.
The
structure of the phage produced by the recombination between two sites flanking
lambda is most interesting. The phage contains two att regions, attL and attR, and is called att2. Such an att2phage
can losethe extra bacterial DNA by a reaction analogous to normal excision
BOP' + POB' -- > POP' + BOB'
This
process requires the Int and Xis proteins and produces a viable lambda phage
genome and a minicircle (Fig. 18.9).
Study of
the excision reaction requires quantitating the input phage λatt2as well
as the product phage. Fortunately, the two may be readilydistinguished. The
concentrations of λatt2 and the λ generated by excision can be
assayed in a mixture of the two by first separating the phage on the basis of
density in equilibrium centrifugation. The λatt2 phage is
more dense than lambda as a result of its additional DNA.
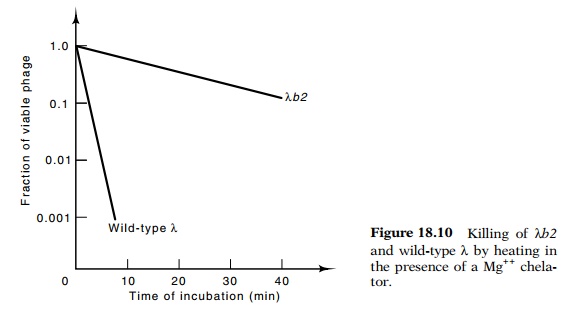
A simpler
assay of the two types of phage makes use of the sensitivity of lambda to heat
and chelators of Mg++. Removal of Mg++ ion from the phage
reduces the charge neutralization between phosphates of the
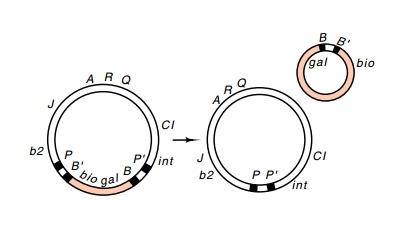
Figure
18.9 An excision re-action between the
two attbio regions onatt2generates
a vi-able phage with a single att
DNA
backbone, and as a result, the DNA expands and can burst the phage coat. Phage
particles possessing less than the usual amount of DNA are more resistant to
heating and removal of Mg++. Combinations of Mg++
chelators and elevated temperatures can be found in which wild-type lambda is
killed by factors of 103 to 104, but the λb2 deletion
mutant is unharmed (Fig. 18.10). The same principle can be used to titer λ in the presence of λatt2. If the
mixture is titered on plates containingpyrophosphate, which chelates magnesium,
only the λ can
proceed through multiple infective cycles and generate plaques. By titering
also on normal plates, the total number of both phage types present can be determined
since both form plaques.
Related Topics