Chapter: Genetics and Molecular Biology: Lambda Phage Integration and Excision
Simultaneous Deletion of Chromosomal and Lambda DNA
Simultaneous Deletion of Chromosomal and Lambda
DNA
The simplest method for attaching lambda to the
host chromosome would be to insert it directly into the DNA. The simplest ideas
are not always correct, however, and in the mid-60s when the question was being
considered, chromosomal DNA seemed sacrosanct. Experiments were designed to
determine whether lambda did integrate into the chromosome or whether it merely
was stuck onto a special place on the chromosome. The genetic demonstration of
lambda’s insertion into the host chromosome utilized deletions. The conclusion
would be that lambda is integrated into the chromosome if a deletion of host
genes extends into the lambda and removes some, but not all, lambda genes.
Often the identification of deletions is difficult.
In this case, however, their identification and isolation were easy. The
nitrate reductase com-plex permits E.
coli to use nitrate as an electron
sink in the absence of
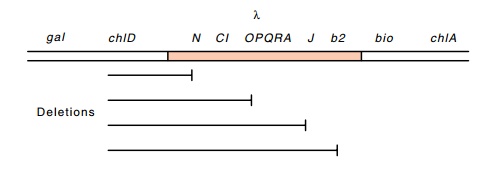
Figure
18.2 Deletions fromchlDinto lambda genes that are
consistent withlambda being directly integrated into the host chromosome.
This enzyme complex is not entirely
specific, and it will reduce chlorate as well. The product, chlorite, however,
is toxic. Such a situ-ation is a geneticist’s dream, for then mutants that are
not killed by the chlorate can easily be isolated. These mutants have lost the
ability to reduce chlorate, and many have been deleted of part or all of the
nitrate reductase genes. Although it would be appropriate to name the genes
involved after nitrate, the genetic locus is frequently called chl for chlorate resistance.
The deletions in the various chl loci frequently extend into adjacent
genes. If chlorate-resistant mutants are isolated in a lambda lysogen, a few
are found to be deletions and some of these are missing some but not all lambda
genes. The pattern of lambda genes remaining or deleted is always consistent
with the lambda genome being linearly incorpo-rated into the chromosome in a
specific orientation with respect to the flanking genes (Fig. 18.2). If some of
the lambda genes have been deleted, how does one show that some lambda genes
remain? The cell certainly cannot produce infective lambda. A method called
marker rescue answers the question. Suppose we wish to test whether a lysogen
with a deletion still has an intact J
gene. A lawn of these cells is poured in soft agar on a plate and a small
volume of a λimm434susJ
(nonsense mutation in J) stock is
spotted onto the lawn. Being heteroimmune, the superinfecting phage is not
repressed even if the deleted lysogen still possesses immunity. A few of these
infecting phage exchange their defective J
gene by recombination for the good one from what remains of the partially
deleted lysogenic lambda. Sufficient phage make this replacement, that cycles
of growth, lysis, and infection of surrounding cells produces a turbid spot on
the opaque lawn. If no J gene is
present on the partially deleted chromosome, the infecting λimm434susJ cannot
proceed through multiple growth cycles, and the lawn is unperturbed. In some
cases, a gene on the partially deleted lysogen will be able to complement the
superinfecting phage as well as recombine with it, but the mapping results are
unaltered. Only if the gene is present in the host cells can the superinfecting
phage grow.
Marker
rescue experiments on chl deletions
revealed many deletions that extended only part way into the lambda genes.
Certainly the simplest explanation for this result is that the DNA of lambda is
con-tiguous with the host DNA.
Related Topics