Chapter: Genetics and Molecular Biology: Lambda Phage Integration and Excision
Isolation of Excision-Deficient Mutants
Isolation of Excision-Deficient Mutants
As
expected, the int mutants can be
helped to lysogenize by complementation. Not surprisingly, these mutants are
also found to excise poorly without assistance. Hence Int protein is also required
for excision. Although it would seem that the process of integration should be
readily reversible with the same components that are used for integration,
surprisingly, a phage-encoded protein is required for excision but not
integration.
A
phenomenon known as heteroimmune curing forms the basis of a simple
demonstration that excision requires a protein in addition to Int protein. If
lambda lysogens are infected with a heteroimmune phage like λimm434, most
cells are lysed, but many of the survivors are found
λ i m m4 3 4 + Lysogen - > Some
nonlysogens
to have
been cured of the lambda. This is called superinfection curing. Apparently the
superinfecting heteroimmune phage provides diffusible products that facilitate
excision of the prophage. Some Int+ deletion phage, but not others,
can promote curing when they superinfect lysogens of different immunity. Thus something in addition to Int protein
must be involved in excision.
The isolation of a nonsense mutation in the gene
required for excision proved that it coded for a protein. This isolation used
heat-pulse curing. If a lysogen of λCI857 growing
at 32° is
heated to 42° for five
minutes and then grown at 32°, the
heat-sensitive CI857
repressor is first denatured, and phage growth begins. Then, after the cells
are cooled to 32°, the
repressor renatures and further phage development ceases before suf-ficient
phage products have accumulated to kill the cells. In the five minutes of
derepression however, sufficient phage proteins are synthe-sized that lambda
can excise from the chromosome. After further cell growth, the excised lambda
genome is diluted away, and daughter cells that are cured of lambda appear at
high frequency.
Heat-pulse
curing was used to isolate excision-defective mutants in the following way
(Fig. 18.5). A mutagenized stock of λCI857 was used
to lysogenize cells. This step selected for phage retaining the ability to
lysogenize. The lysogenic cells resulting from this step were grown at 32° and then replicated onto three
petri plates. One was incubated at 32°, one was incubated at 42°, and the third was incubated at
42° for five
minutes, at 32° for two
hours, and then at 42°
overnight.
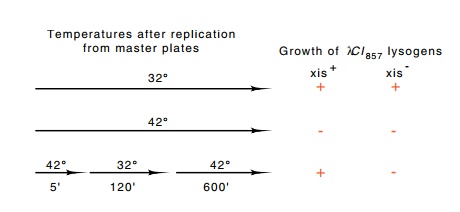
Figure
18.5 Temperature protocols for the
identification ofxis+andxis-by theinability of the latter to
excise following a short heat induction. Each of the three temperature time
lines represents the conditions the three petri plates were exposed to.
The first plate kept viable copies of all the colonies. The second plate showed which colonies were infected with lambda, as growth at 42° would induce the phage and
kill lysogens. Growth on the third plate indicated which colonies could
heat-pulse cure. On this plate, the lambda lysogens would heat-pulse cure, and
the cured cells in such a colony would be capable of growing into colonies
during the subsequent growth at 42°. Any
colony whose cells possessed excision-defective phage could not heat-pulse cure
and would therefore be killed by the attempted growth of the unexcised prophage
during the subsequent extended exposure to 42°.
Later, a
simpler method for detecting excision-defective mutants was devised. This
scheme uses cells in which lambda has mistakenly inte-grated into a site within
the gal genes that resembles the
authentic attB site. The cells are
Gal- as a result. Infection of these cells by heteroim-mune phage
able to provide Int and Xis proteins in trans
catalyzes excision of the phage from the gal
genes, and the cells become Gal+. If such infected cells are plated
on galactose plates, plaques with Gal+ revertants are red on medium
containing galactose indicator dye and Gal- plaques are white. With
this convenient assay, the excision abilities of many different phage can be
assayed on a single galactose indicator plate.
Using the
plate assay for excision, Enquist and Weisberg performed a thorough genetic
analysis of the att-int-xis region of
the lambda chromosome. They isolated and characterized hundreds of int and xis point mutations. The mutations fell into two complementation
groups indicating that two genes were involved. One codes for the Int protein
and one for the Xis protein. The mutations were mapped with a set of deletions
ending in the region. The large number of mutants studied permitted a
reasonable estimation of the sizes of the genes. The int gene appeared to be about 1,240 base pairs long, and the xis gene was very small, only 110 base
pairs long. No additional phage genes acting in trans and directly involved in the integration or excision process
werediscovered.
Related Topics