Chapter: Clinical Anesthesiology: Clinical Pharmacology: Pharmacological Principles
Theories of Anesthetic Action
Pharmacodynamics of Inhalation Anesthetics
THEORIES OF ANESTHETIC ACTION
General anesthesia is an altered
physiological state characterized by reversible loss of consciousness,
analgesia, amnesia, and some degree of muscle relaxation. The multitude of
substances capable of producing general anesthesia is remarkable: inert
elements (xenon), simple inorganic compounds (nitrous oxide), halogenated
hydrocarbons (halo-thane), ethers (isoflurane, sevoflurane, desflurane), and
complex organic structures (propofol). A unify-ing theory explaining anesthetic
action would have to accommodate this diversity of structure. In fact, the
various agents probably produce anesthesia by differing sets of molecular
mechanisms. Inhalational agents interact with numerous ion channels present in
the CNS and peripheral nervous system. Nitrous oxide and xenon are believed to
inhibit N-methyl-D-aspartate (NMDA)
receptors. NMDA recep-tors are excitatory receptors in the brain. Other
inhalational agents may interact at other receptors(eg, gamma-aminobutyric acid
[GABA]-activated chloride channel conductance) leading to anesthetic effects.
Additionally, some studies suggest that inha-lational agents continue to act in
a nonspecific man-ner, thereby affecting the membrane bilayer. It is possible
that inhalational anesthetics act on multiple protein receptors that block
excitatory channels and promote the activity of inhibitory channels affect-ing
neuronal activity, as well as by some nonspecific membrane effects.
Th ere does not seem to be a single
macroscopic site of action that is shared by all inhalation agents. Specific
brain areas affected by various anesthetics include the reticular activating
system, the cerebral cortex, the cuneate nucleus, the olfactory cortex, and the
hippocampus; however, to be clear, general anes-thetics bind throughout the
CNS. Anesthetics have also been shown to depress excitatory transmission in the
spinal cord, particularly at the level of the dor-sal horn interneurons that
are involved in pain trans-mission. Differing aspects of anesthesia may be
related to different sites of anesthetic action. For example, unconsciousness
and amnesia are probably mediated by cortical anesthetic action, whereas the
suppression of purposeful withdrawal from pain may be related to subcortical
structures, such as the spinal cord or brain stem. One study in rats revealed
that removal of the cerebral cortex did not alter the potency of the
anesthetic! Indeed, measures of mini-mal alveolar concentration (MAC), the
anesthetic concentration that prevents movement in 50% of subjects or animals,
are dependent upon anesthetic effects at the spinal cord and not at the cortex.
Past understanding of anesthetic action
attempted to identify a unitary hypothesis ofanesthetic effects. This
hypothesis proposes that all inhalation agents share a common mechanism of
action at the molecular level. This was previously supported by the observation
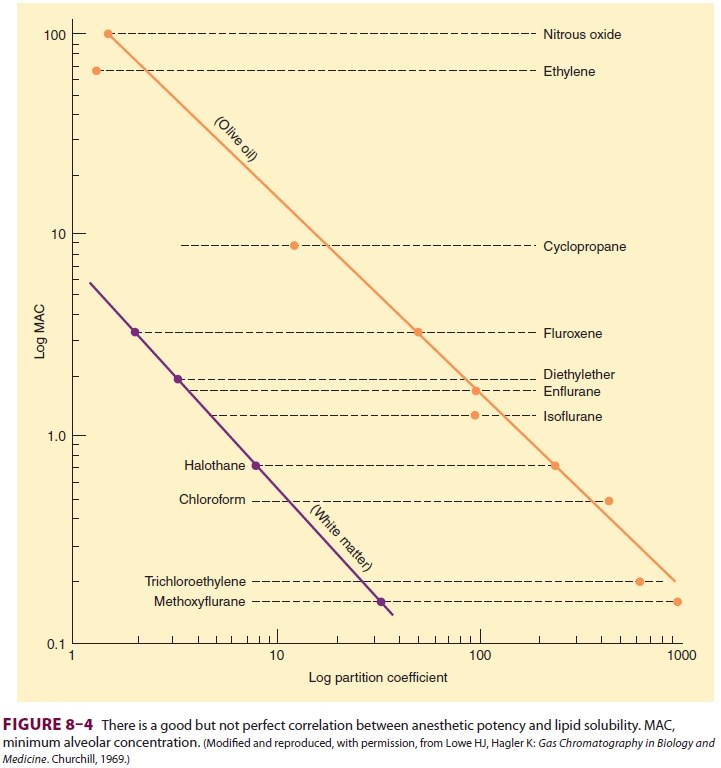
that the anesthetic potency of inhalation agents correlates directly with their
lipid solubility (Meyer–Overton rule). The implication is that anesthesia
results from molecules dissolving at specific lipophilic sites. Of course, not
all lipid-soluble molecules are anesthetics (some are actually convulsants),
and the correlation between anesthetic potency and lipid solubility is only
approximate (Figure
8–4).
Neuronal membranes contain a multitude
of hydrophobic sites in their phospholipid bilayer. Anesthetic binding to these
sites could expand the bilayer beyond a critical amount, altering membrane
function (critical volume hypothesis). Although this theory is almost certainly
an oversimplification, it explains an interesting phenomenon: the reversal of
anesthesia by increased pressure. Laboratory animals exposed to elevated
hydrostatic pressure develop a resistance to anesthetic effects. Perhaps the
pres-sure is displacing a number of molecules from the membrane or distorting
the anesthetic binding sites in the membrane, increasing anesthetic
require-ments. However, studies in the 1980s demonstrated the ability of
anesthetics to inhibit protein actions, shifting attention to the numerous ion
channels that might affect neuronal transmission and away from the critical
volume hypothesis.
General anesthetic action could be due
to alter-ations in any one (or a combination) of several cel-lular systems,
including voltage-gated ion channels, ligand-gated ion channels, second
messenger func-tions, or neurotransmitter receptors. For example, many
anesthetics enhance GABA inhibition of the CNS. Furthermore, GABA receptor
agonists seem to enhance anesthesia, whereas GABA antagonists reverse some
anesthetic effects. There seems to be a strong correlation between anesthetic potency
and potentiation of GABA receptor activity. Thus, anesthetic action may relate
to binding in relatively hydrophobic domains in channel proteins (GABA
receptors). Modulation of GABA function may prove to be a principal mechanism
of action for many anesthetic drugs.
The glycine receptor α1-subunit, whose func-tion is enhanced by
inhalation anesthetics, is another potential anesthetic site of action.
The tertiary and quaternary structure of
amino acids within an anesthetic-binding pocket could be modified by inhalation
agents, perturbing the receptor itself, or indirectly producing an effect at a
distant site.
Other ligand-gated ion channels whose
modula-tion may play a role in anesthetic action include nico-tinic
acetylcholine receptors and NMDA receptors.
Investigations into mechanisms of anesthetic action are likely to remain ongoing for many years, as many protein channels may be affected by indi-vidual anesthetic agents, and no obligatory site has yet been identified. Selecting among so many molecular targets for the one(s) that provide opti-mum effects with minimal adverse actions will be the challenge in designing better inhalational agents.
Related Topics