Chapter: Clinical Anesthesiology: Clinical Pharmacology: Pharmacological Principles
Factors Affecting Alveolar Concentration
FACTORS AFFECTING ALVEOLAR CONCENTRATION FA
Uptake
If there were no uptake of anesthetic
agent by the body, the alveolar gas concentration (Fa) would rapidly approach
the inspired gas concentration (Fi). Because anesthetic agents are taken up by
the pulmonary circulation during induction, alveolar concentrations lag behind
inspired concentrations (Fa/Fi <1.0). The
greater the uptake, the slower the rate of rise of the alveolar concentration
and the lower the Fa:Fi ratio.
Because the concentration of a gas is
directly proportional to its partial pressure, the alveolar par-tial pressure
will also be slow to rise. The alveolar partial pressure is important because
it determines the partial pressure of anesthetic in the blood and, ultimately,
in the brain. Similarly, the partial pres-sure of the anesthetic in the brain
is directly propor-tional to its brain tissue concentration, which determines
clinical effect.
Therefore, the greater the uptake of
anesthetic agent, the greater the difference betweeninspired and alveolar
concentrations, and the slower the rate of induction.
Three factors affect anesthetic uptake:
solubil-ity in the blood, alveolar blood flow, and thedifference in partial
pressure between alveolar gas and venous blood.
Relatively soluble agents, such as
nitrous oxide, are taken up by the blood less avidly than more solu-ble agents,
such as halothane. As a consequence, the alveolar concentration of nitrous
oxide rises faster than that of halothane, and induction is faster. The
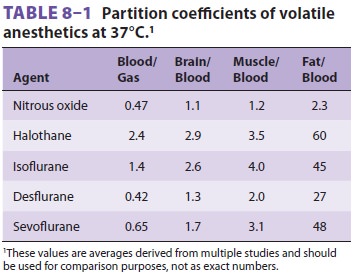
relative solubilities of an anesthetic in air, blood, and tissues are expressed
as partition coefficients (Table 8–1). Each coefficient is the ratio of
the concentrations of the anesthetic gas in each of two phases at steady state.
Steady state is defined as equal partial pressures in the two phases. For
instance, the blood/gas partition coefficient (λb/g) of nitrous oxide at 37°C is 0.47. In other words,
at steady state, 1 mL of blood contains 0.47 as much nitrous oxide as does 1 mL
of alveolar gas, even though the partial pressures are the same. Stated another
way, blood has 47% of the capacity for nitrous oxide as alveolar gas. Nitrous
oxide is much less soluble in blood than is halothane, which has a blood/gas
partition coef-ficient at 37°C of 2.4. Thus, almost five times more halothane
than nitrous oxide must be dissolved to raise the partial pressure of blood.
The higher the blood/gas coefficient, the greater the anesthetic’s sol-ubility
and the greater its uptake by the pulmonary circulation. As a consequence of
this increased solu-bility, alveolar partial pressure rises more slowly, and
induction is prolonged. Because fat/blood partition coefficients are greater
than 1, blood/gas solubility is increased by postprandial lipidemia and is
decreased by anemia.
The second factor that affects uptake is
alveolar blood flow, which—in the absence of pulmonary shunting—is essentially
equal to cardiac output. If the cardiac output drops to zero, so will
anesthetic uptake. As cardiac output increases, anesthetic uptake increases,
the rise in alveolar partial pressure slows, and induction is delayed. The
effect of chang-ing cardiac output is less pronounced for insoluble
anesthetics, as so little is taken up regardless of alveolar blood flow.
Low-output states predispose patients to overdosage with soluble agents, as the
rate of rise in alveolar concentrations will be markedly increased.
The final factor affecting uptake of
anesthetic by the pulmonary circulation is the partial pressure dif-ference
between alveolar gas and venous blood. This gradient depends on tissue uptake.
If anesthetics did not pass into organs such as the brain, venous and alveolar
partial pressures would become identical, and there would be no pulmonary
uptake. The trans-fer of anesthetic from blood to tissues is determined by
three factors analogous to systemic uptake: tissue solubility of the agent
(tissue/blood partition coeffi-cient), tissue blood flow, and the difference in
partial pressure between arterial blood and the tissue.
To better understand inhaled anesthetic
uptake and distribution, tissues have been classified into four groups based on
their solubility and blood flow (Table 8–2). The highly perfused vessel-rich
group (brain, heart, liver, kidney, and endocrine organs) is
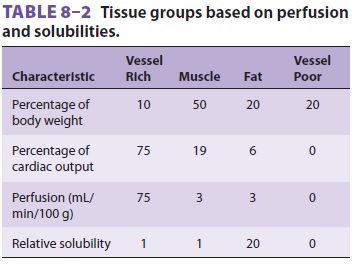
the first to encounter appreciable
amounts of anes-thetic. Moderate solubility and small volume limit the capacity
of this group, so it is also the fi rst to reach steady state (ie, arterial and
tissue partial pressures are equal). The muscle group (skin and muscle) is not
as well perfused, so uptake is slower. In addition, it has a greater capacity
due to a larger volume, and uptake will be sustained for hours. Perfusion of
the fat group nearly equals that of the muscle group, but the tremendous
solubility of anesthetic in fat leads to a total capacity (tissue/blood
solubility × tissue vol-ume) that would take days to approach
steady state. The minimal perfusion of the vessel-poor group (bones, ligaments,
teeth, hair, and cartilage) results in insignificant uptake.
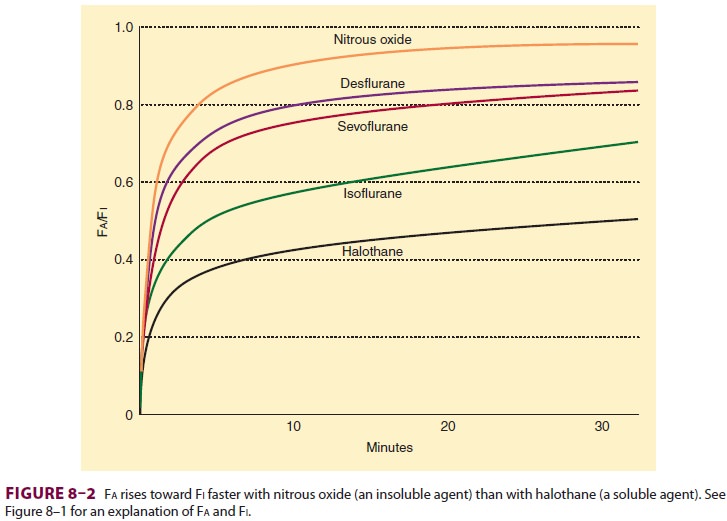
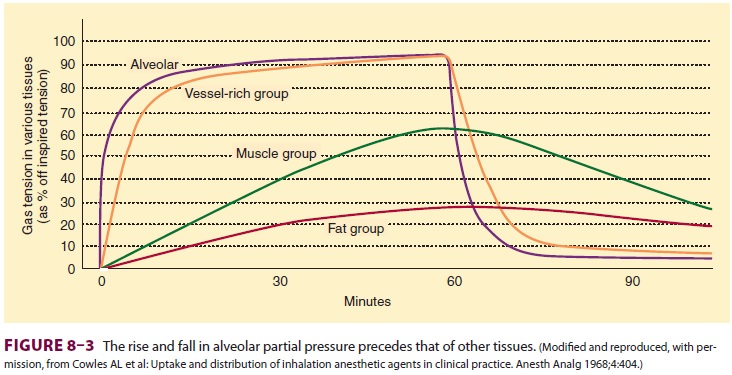
Anesthetic uptake produces a
characteristic curve that relates the rise in alveolar concentra-tion to time (Figure 8–2).
The shape of this graph is determined by the uptakes of individual tissue
groups (Figure
8–3). The initial steep rate of uptake is due to unopposed filling
of the alveoli by ventila-tion. The rate of rise slows as the vessel-rich
group— and eventually the muscle group—approach steady state levels of
saturation.
Ventilation
The lowering of alveolar partial pressure by uptake can be countered by increasing alveolar ventila-tion. In other words, constantly replacing anesthetic taken up by the pulmonary bloodstream results in better maintenance of alveolar concentration. The effect of increasing ventilation will be most obvious in raising the Fa/Fi for soluble anesthetics, as they are more subject to uptake. Because the Fa/Fi very rapidly approaches 1.0 for insoluble agents, increas-ing ventilation has minimal effect. In contrast to the effect of anesthetics on cardiac output, anesthetics that depress spontaneous ventilation (eg, ether or halothane) will decrease the rate of rise in alveolar concentration and create a negative feedback loop.
Concentration
The slowing of induction due to uptake
from alve-olar gas can be reduced by increasing the inspired concentration.
Interestingly, increasing the inspired concentration not only increases the
alveolar con-centration, but also increases its rate of rise (ie, increases
Fa/Fi), because of two phenomena (see Figure 8–1) that produce a so-called
“concentrating effect.” First, if 50% of an anesthetic is taken up by the
pulmonary circulation, an inspired concentra-tion of 20% (20 parts of
anesthetic per 100 parts of gas) will result in an alveolar concentration of
11% (10 parts of anesthetic remaining in a total volume of 90 parts of gas). On
the other hand, if the inspired concentration is raised to 80% (80 parts of
anesthetic per 100 parts of gas), the alveolar concentration will be 67% (40
parts of anesthetic remaining in a total volume of 60 parts of gas). Thus, even
though 50% of the anesthetic is taken up in both examples, a higher inspired
concentration results in a disproportion-ately higher alveolar concentration.
In this example, increasing the inspired concentration 4-fold results in a
6-fold increase in alveolar concentration. The extreme case is an inspired
concentration of 100% (100 parts of 100), which, despite a 50% uptake, will
result in an alveolar concentration of 100% (50 parts of anesthetic remaining
in a total volume of 50 parts of gas).
The second phenomenon responsible for
the concentration effect is the augmented inflow effect. Using the example
above, the 10 parts of absorbed gas must be replaced by an equal volume of the
20% mixture to prevent alveolar collapse. Thus, the alveo-lar concentration
becomes 12% (10 plus 2 parts of anesthetic in a total of 100 parts of gas). In
contrast, after absorption of 50% of the anesthetic in the 80% gas mixture, 40
parts of 80% gas must be inspired. This further increases the alveolar
concentration from 67% to 72% (40 plus 32 parts of anesthetic in a volume of
100 parts of gas).
The concentration effect is more
significant with nitrous oxide, than with the volatile anes-thetics, as the
former can be used in much higher concentrations. Nonetheless, a high
concentration of nitrous oxide will augment (by the same mech-anism) not only
its own uptake, but theoretically that of a concurrently administered volatile
anes-thetic. The concentration effect of one gas upon another is called the
second gas effect, which is probably insignificant in the clinical practice of
anesthesiology.
Related Topics