Chapter: Clinical Anesthesiology: Clinical Pharmacology: Pharmacological Principles
Sevoflurane - Clinical Pharmacology of Inhalation Anesthetics
SEVOFLURANE
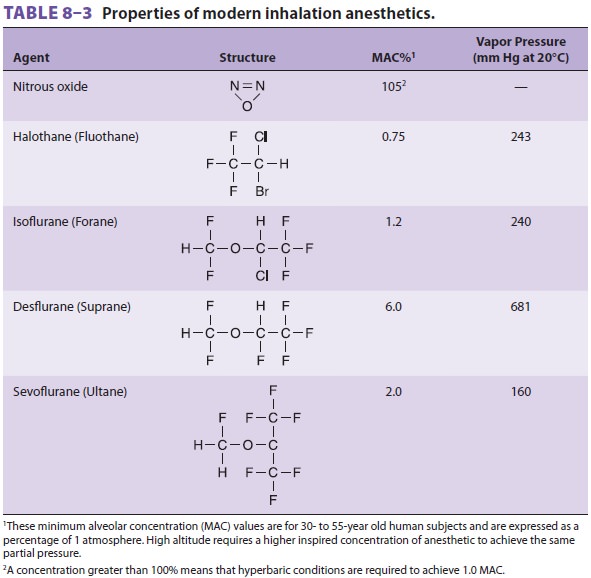
Physical Properties
Like desflurane, sevoflurane is
halogenated with fluorine. Sevoflurane’s solubility in blood is sligh-tly
greater than desflurane (λb/g 0.65 versus 0.42) (see Table 8–3). Nonpungency and
rapid increases in alveolar anesthetic concentration make sevoflurane an
excellent choice for smooth and rapid inhalation inductions in pediatric and
adult patients. In fact, inhalation induction with 4% to 8% sevoflurane in a
50% mixture of nitrous oxide and oxygen can be achieved within 1 min. Likewise,
its low blood solubility results in a rapid fall in alveolar anesthetic
concentration upon dis-continuation and a more rapid emergence com-pared with
isoflurane (although not an earlier discharge from the post-anesthesia care
unit). Sevoflurane’s modest vapor pressure permits the use of a conventional
variable bypass vaporizer.
Effects on Organ Systems
A. Cardiovascular
Sevoflurane mildly depresses myocardial
contractil-ity. Systemic vascular resistance and arterial blood pressure
decline slightly less than with isoflurane or desflurane. Because sevoflurane
causes little, if any, rise in heart rate, cardiac output is not maintained as
well as with isoflurane or desflurane. Sevoflurane may prolong the QT interval,
the clinical signifi-cance of which is unknown. QT prolongation may be manifest
60 min following anesthetic emergence in infants.
B. Respiratory
Sevoflurane depresses respiration and
reverses bronchospasm to an extent similar to that of isoflurane.
C. Cerebral
Similar to isoflurane and desflurane,
sevoflurane causes slight increases in CBF and intracranial pressure at
normocarbia, although some studies show a decrease in cerebral blood flow. High
con-centrations of sevoflurane (>1.5 MAC) may
impair autoregulation of CBF, thus allowing a drop in CBF during hemorrhagic hypotension.
This effect on CBF autoregulation seems to be less pronounced than with
isoflurane. Cerebral metabolic oxygen requirements decrease, and seizure
activity has not been reported.
D. Neuromuscular
Sevoflurane produces adequate muscle
relaxation for intubation of children following an inhalation induction.
E. Renal
Sevoflurane slightly decreases renal
blood flow. Its metabolism to substances associated with impaired renal tubule
function (eg, decreased concentrating ability) is discussed below.
F. Hepatic
Sevoflurane decreases portal vein blood
flow, but increases hepatic artery blood flow, thereby main-taining total
hepatic blood flow and oxygen delivery. It is generally not associated with
immune-mediated anesthetic hepatotoxicity
Biotransformation & Toxicity
The liver microsomal enzyme P-450
(specifically the 2E1 isoform) metabolizes sevoflurane at a rate one-fourth
that of halothane (5% versus 20%), but 10 to 25 times that of isoflurane or
desflurane and may be induced with ethanol or phenobarbital pretreat-ment. The
potential nephrotoxicity of the resulting rise in inorganic fluoride (F−) was discussed earlier. Serum fluoride
concentrations exceed 50 µmol/L in approximately 7% of patients who receive
sevoflu-rane, yet clinically significant renal dysfunction has not been
associated with sevoflurane anesthesia. The overall rate of sevoflurane
metabolism is 5%, or 10 times that of isoflurane. Nonetheless, there has been
no association with peak fluoride levels following sevoflurane and any renal
concentrating abnormality.
Alkali such as barium hydroxide lime or
soda lime (but not calcium hydroxide) can degrade sevoflurane, producing
another proven (at least in rats) nephrotoxic end product (compound A, flu-oromethyl-2,2-difluoro-1-[trifluoromethyl]vinyl
ether). Accumulation of compound A increases with increased respiratory gas
temperature, low-flow anesthesia, dry barium hydroxide absorbent (Baralyme),
high sevoflurane concentrations, and anesthetics of long duration.
Most studies have not associated sevoflurane
with any detectable postoperative impairment of renal function that would
indicate toxicity or injury. Nonetheless, some clinicians recommend that fresh
gas flows be at least 2 L/min for anesthet-ics lasting more than a few hours
and that sevoflu-rane not be used in patients with preexisting renal
dysfunction. Sevoflurane can also be degraded into hydro-gen fluoride by metal
and environmental impurities present in manufacturing equipment, glass bottle
packaging, and anesthesia equipment. Hydrogen fluoride can produce an acid burn
on contact with respiratory mucosa. The risk of patient injury has been
substantially reduced by inhibition of the deg-radation process by adding water
to sevoflurane during the manufacturing process and packaging it in a special
plastic container. The manufacturer has also distributed a “Dear Provider”
letter warning of isolated incidents of fire in the respiratory circuits of
anesthesia machines with desiccated CO2
absorbent when sevoflurane was used.
Contraindications
Contraindications include severe
hypovolemia, sus-ceptibility to malignant hyperthermia, and intracra-nial
hypertension.
Drug Interactions
Like other volatile anesthetics,
sevoflurane potenti-ates NMBAs. It does not sensitize the heart to
cate-cholamine-induced arrhythmias.
Related Topics