Chapter: Clinical Anesthesiology: Clinical Pharmacology: Pharmacological Principles
Nitrous Oxide - Clinical Pharmacology of Inhalation Anesthetics
Clinical Pharmacology of Inhalation Anesthetics
NITROUS OXIDE
Physical Properties
Nitrous oxide (N2O; laughing gas) is colorless and essentially
odorless. Although nonexplosive and nonflammable, nitrous oxide is as capable
as oxygen of supporting combustion. Unlike the potent volatile
agents, nitrous oxide is a gas at room
temperature and ambient pressure. It can be kept as a liquid under pressure
because its critical temperature lies above room temperature. Nitrous oxide is
a relatively inex-pensive anesthetic; however, concerns regarding its safety
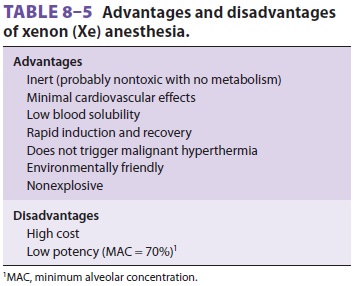
have led to continued interest in alternatives such as xenon (Table 8–5). As noted earlier, nitrous oxide,
like xenon, is an NMDA receptor antagonist.
Effects on Organ Systems
A. Cardiovascular
Nitrous oxide has a tendency to
stimulate the sym-pathetic nervous system. Thus, even though nitrous oxide
directly depresses myocardial contractility in
vitro, arterial blood pressure, cardiac
output, and heart rate are essentially unchanged or slightly ele-vated in vivo
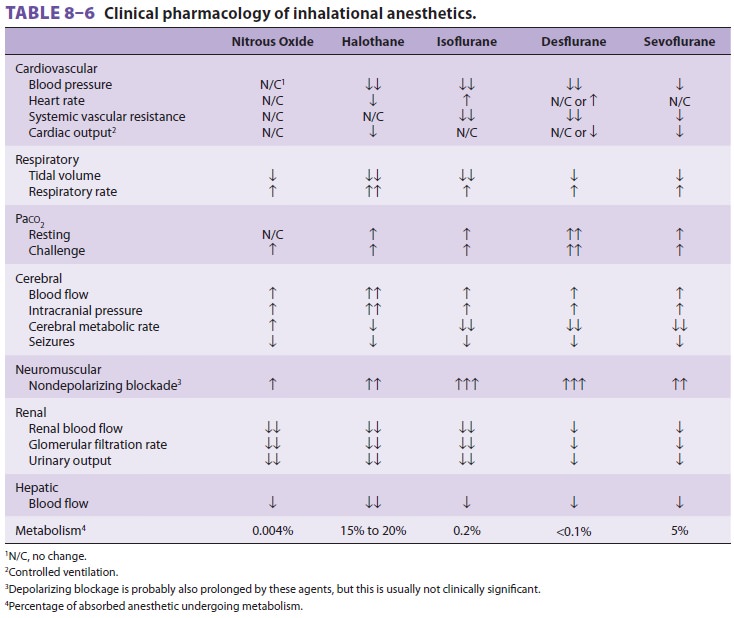
because of its stimulation of catechol-amines (Table 8–6). Myocardial
depression may be unmasked in patients with coronary artery disease or severe
hypovolemia. Constriction of pulmonary vascular smooth muscle increases
pulmonary vas-cular resistance, which results in a generally modest elevation
of right ventricular end-diastolic pressure. Despite vasoconstriction of
cutaneous vessels, periph-eral vascular resistance is not significantly
altered.
B. Respiratory
Nitrous oxide increases respiratory rate
(tachy-pnea) and decreases tidal volume as a result of CNS stimulation and,
perhaps, activation of pulmonary stretch receptors. The net effect is a minimal
change in minute ventilation and resting arterial CO 2 levels. Hypoxic drive, the ventilatory response to
arterial hypoxia that is mediated by peripheral chemorecep-tors in the carotid
bodies, is markedly depressed by even small amounts of nitrous oxide. This is a
con-cern in the recovery room.
C. Cerebral
By increasing CBF and cerebral blood
volume, nitrous oxide produces a mild elevation of intracra-nial pressure.
Nitrous oxide also increases cerebral oxygen consumption (CMRO2). These two effects make nitrous oxide
theoretically less attractive than other agents for neuroanesthesia.
Concentrations of nitrous oxide below MAC may provide analgesia in dental
surgery, labor, traumatic injury, and minor surgical procedures.
D. Neuromuscular
In contrast to other inhalation agents,
nitrous oxide does not provide significant muscle relaxation. In fact, at high
concentrations in hyperbaric cham-bers, nitrous oxide causes skeletal muscle
rigidity. Nitrous oxide is not a triggering agent of malignant hyperthermia.
E. Renal
Nitrous oxide seems to decrease renal
blood flow by increasing renal vascular resistance. This leads to a drop in
glomerular filtration rate and urinary output.
F. Hepatic
Hepatic blood flow probably falls during
nitrous oxide anesthesia, but to a lesser extent than with the volatile agents.
G. Gastrointestinal
Use of nitrous oxide in adults increases
the risk of postoperative nausea and vomiting, presumably as a result of
activation of the chemoreceptor trigger zone and the vomiting center in the
medulla.
Biotransformation & Toxicity
During emergence, almost all nitrous
oxide is eliminated by exhalation. A small amount diffuses out through the
skin. Biotransformation is limited to the less than 0.01% that undergoes
reductive metabolism in the gastrointestinal tract by anaero-bic bacteria.
By irreversibly oxidizing the cobalt
atom in vitamin B12, nitrous oxide inhibits enzymes that
are vitamin B12 dependent. These enzymes include
methionine synthetase, which is necessary for myelin formation, and thymidylate
synthetase, which is necessary for DNA synthesis. Prolonged expo-sure to
anesthetic concentrations of nitrousoxide can result in bone marrow depression
(mega-loblastic anemia) and even neurological deficiencies (peripheral
neuropathies). However, administration of nitrous oxide for bone marrow harvest
does not seem to affect the viability of bone marrow mono-nuclear cells.
Because of possible teratogenic effects, nitrous oxide is often avoided in
pregnant patients who are not yet in the third trimester. Nitrous oxide may
also alter the immunological response to infec-tion by affecting chemotaxis and
motility of poly-morphonuclear leukocytes.
Contraindications
Although nitrous oxide is insoluble in
comparison with other inhalation agents, it is 35 times more soluble than
nitrogen in blood. Thus, it tends to diffuse into air-containing cavities more
rapidly than nitrogen is absorbed by the bloodstream. For instance, if a
patient with a 100-mL pneumothorax inhales 50% nitrous oxide, the gas content
of the pneumothorax will tend to approach that of the bloodstream. Because
nitrous oxide will diffuse into the cavity more rapidly than the air
(princi-pally nitrogen) diffuses out, the pneumothorax expands until it contains
100 mL of air and 100 mL of nitrous oxide. If the walls surrounding the cavity
are rigid, pressure rises instead of volume.
Examples of conditions in which nitrous
oxide might be hazardous include venous or arterial air embolism, pneumothorax,
acute intestinal obstruction with bowel distention, intracranial air
(pneumocephalus following dural closure or pneumoencephalography), pulmonary
air cysts, intraocular air bubbles, and tympanic mem-brane grafting. Nitrous
oxide will even diffuse into tracheal tube cuffs, increasing the pressure
against the tracheal mucosa. Obviously, nitrous oxide is of limited value in
patients requiring high inspired oxygen concentrations.
Drug Interactions
Because the high MAC of nitrous oxide
prevents its use as a complete general anesthetic, it is frequently used in
combination with the more potent volatile agents. The addition of nitrous oxide
decreases the requirements of these other agents (65% nitrous oxide decreases
the MAC of the volatile anesthet-ics by approximately 50%). Although nitrous
oxide should not be considered a benign carrier gas, it does attenuate the
circulatory and respiratory effects of volatile anesthetics in adults. Nitrous
oxide potentiates neuromuscular blockade, but less so than the volatile agents.
The concentration of nitrous oxide flowing through a vaporizer can influence
the concentration of volatile anesthetic delivered. For example, decreasing
nitrous oxide concentration (ie, increasing oxygen concentration) increases the
concentration of volatile agent despite a constant vaporizer setting. This
disparity is due to the relative solubilities of nitrous oxide and oxygen in
liquid vol-atile anesthetics. The second gas effect was discussed earlier.
Nitrous oxide is an ozone-depleting gas with greenhouse effects.
Related Topics