Ionic Equilibrium | Chemistry - The pH scale | 12th Chemistry : UNIT 8 : Ionic Equilibrium
Chapter: 12th Chemistry : UNIT 8 : Ionic Equilibrium
The pH scale
The pH scale
We usually deal with acid / base solution in the concentration range 10-1
to 10-7M . To express the strength of such low concentrations,
Sorensen introduced a logarithmic scale known as the pH scale. The term pH is
derived from the French word ŌĆśPurissance
de hydrogeneŌĆÖ meaning, the power of hydrogen. pH of a solution is defined
as the negative logarithm of base 10 of the molar concentration of the
hydronium ions present in the solution.
pH = - log 10[H3O+ ] .....(8.5)
The concentration of H3O+ in a solution of known
pH can be calculated using the following expression.
[H3O+] = 10-pH
(or) [H3O+] = antilog of (-pH) .....(8.6)
Similarly, pOH can also be defined as follows
pOH = - log 10[OH-] .....(8.7)
As discussed earlier, in neutral solutions, the concentration of [H3O+
] as well as [OH+] is equal to 1 ├Ś 10-7 M at 25┬║C . The pH of a neutral solution can be calculated
by substituting this H3O+ concentration in the expression
(8.5)
pH = - log10 [H3O+]
= - log 10 10-7
= (-7)(-1) log1010= +7 (1)= 7 { [ log1010
=1] }
Similary, we can calculate the pOH of a neutral solution using the
expression (8.7), it is also equal to 7.
The negative sign in the expression (8.5) indicates that when the
concentration of [H3O+ ] increases the pH value decreases.
For example, if the [H3O+ ] increases from to 10-7
to10-5 M , the pH value of the solution decreases from 7 to 5. We
know that in acidic solution, [H3O+ ] > [OH-
] , i.e., [H3O+ ] > 10-7 . Similarly in basic solution [H3O+
] < 10-7. So, we can conclude that acidic solution should have pH
value less than 7 and basic solution should have pH value greater than 7.
Relation between pH and pOH
A relation between pH and pOH can be established using their following
definitions
pH = -log10[H3O+] .....(8.5)
pOH = - log10[OH-] .....(8.7)
Adding equation (8.5) and (8.7)

pH + pOH = - log10[H3O+] - log10[OH-]
= - ( log10[H3O+]
+ log10[OH-])
pH+pOH = -log10[H3O+][OH-] [log a+logb = logab]
We know that [H3O+][OH-]=Kw
ŌćÆ pH + pOH = -log10 Kw
ŌćÆ pH+pOH = pKw [pKw = -log10 Kw]
at 25┬║ C, the
ionic product of water, Kw =1 ├Ś 10-14
pKw = - log1010-14
= 14 log1010
= 14
Ōł┤ (8.7) ŌćÆ Ōł┤ At 25┬║ C, pH + pOH= 14
Example 8.2
Calculate
the pH of 0.001M HCl solution
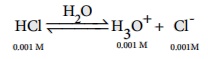
H3O+ from the auto ionisation of H2O
(10-7M) is negligible when compared to the H3O+
from 10-3M HCl.
Hence [H3O+ ]= 0.001 mol dmŌĆō3
pH = -log10 [H3O+]
= -log10 (0.001)
= -log10 (10-3 ) = 3
Note: If the concentration of the acid or
base is less than 10ŌĆō6 M,
the concentration of H3O+
produced due to the auto ionisation of water cannot be negleted and in such
cases
[H3O+] = 10-7(from water)
+ [H3O+] (from the acid)
similarly, [OH-] = 10-7 M (from
water) + [OH-] (from the base)
Example 8.3
Calculate
pH of 10-7 M HCl
If we do not consider [H3O+ ] from the ionisation
of H2O,
then [H3O+ ] = [HCl] = 10-7M
i.e., pH = 7, which is a pH of a neutral solution. We know that HCl
solution is acidic whatever may be the concentration of HCl i.e, the pH value
should be less than 7. In this case the concentration of the acid is very low
(10-7M) Hence, the H3O+ (10-7M)
formed due to the auto ionisation of water cannot be neglected.
so, in this case we should consider [H3O+ ] from
ionisation of H 2O
[H3O+ ] = 10-7 (from HCl) + 10-7
(from water)
= 10-7 (1+1)
= 2 ├Ś10-7
pH = -log10[H3O+ ]
= - log 2 - (-7).log10 10
= 7-log 2
= 7-0.3010 = 6.6990
= 6.70
Evaluate yourself ŌĆō 6
a. Calculate pH of 10-8M H2 SO4
b. Calculate the concentration of hydrogen ion in moles
per litre of a solution whose pH is 5.4
c. Calculate the pH of an aqueous solution obtained by
mixing 50ml of 0.2 M HCl with 50ml 0.1 M NaOH
a) Answer
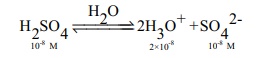
In this case the concentration of H2SO4
is very low and hence [H3O+ ] from water cannot be neglected
Ōł┤[H3O+]
= 2 ├Ś10-8 (from H2SO4 ) + 10-7
(from water)
= 10-8
(2+10)
= 12├Ś10-8
= 1.2 ├Ś10-7
pH = - log10[H3O+]
= - log10 (1.2 ├Ś 10-7)
= 7 - log101.2
= 7 -0.0791
= 6.9209
b) Answer
pH of the solution
= 5.4
[H3O+]
= antilog of (-pH)
= anitlog of (-5.4)
= antilog of (-6 + 0.6) = 6.6
= 3.981├Ś10-6
i.e., 3.98 ├Ś 10-6 mol dm-3
c) Answer
No of moles of HCl
= 0.2├Ś 50 ├Ś 10-3 = 10 ├Ś 10-3
No of moles of NaOH
= 0.1 ├Ś 50 ├Ś 10-3 = 5 ├Ś 10-3
No of moles of HCl
after mixing = 10 ├Ś 10-3 - 5 ├Ś 10-3
= 5 ├Ś10-3
after mixing total
volume = 100mL
Ōł┤ Concentration of HCl in moles per litre = 5├Ś10-3mole / 100├Ś10-3L
[H3O+]
= 5 ├Ś10-2 M
pH = - log (5 ├Ś 10-2
)
= 2 - log 5
= 2 - 0.6990
= 1.30
Related Topics