Ionic Equilibrium | Chemistry - Acids and bases | 12th Chemistry : UNIT 8 : Ionic Equilibrium
Chapter: 12th Chemistry : UNIT 8 : Ionic Equilibrium
Acids and bases
Acids and bases
The term ŌĆśacidŌĆÖ is derived from the latin word ŌĆśacidusŌĆÖ meaning sour. We have already learnt in earlier classes
that acid tastes sour, turns the blue litmus to red and reacts with metals such
as zinc and produces hydrogen gas. Similarly base tastes bitter and turns the
red litmus to blue.
These classical concepts are not adequate to explain the complete
behaviour of acids and bases. So, the scientists developed the acid ŌĆō base
concept based on their behaviour.
Let us, learn the concept developed by scientists Arrhenius, Bronsted
and Lowry and Lewis to describe the properties of acids and bases.
Arrhenius Concept
One of the earliest theories about acids and bases was proposed by
swedish chemist Svante Arrhenius. According to him, an acid is a substance that
dissociates to give hydrogen ions in water. For example, HCl, H2SO4
etc., are acids. Their dissociation in aqueous solution is expressed as
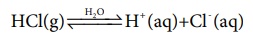
The H+ ion in aqueous solution is highly hydrated and usually
represented as H3O+ , the simplest hydrate of proton [H(H2O)
]+ . We use
both H+and H3O+ to mean the same.
Similarly a base is a substance that dissociates to give hydroxyl ions
in water. For example, substances like NaOH, Ca(OH)2 etc., are
bases.
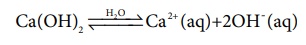
Limitations of Arrhenius concept
i. Arrhenius theory does not explain the behaviour of acids and bases in
non aqueous solvents such as acetone, Tetrahydrofuran etc...
ii. This theory does not account for the basicity of the substances like
ammonia (NH3 ) which do not possess hydroxyl group.
Evaluate yourself ŌĆō 1
Classify the following as acid (or) base using Arrhenius concept
i)HNO3 ii) Ba(OH)2 iii) H3 PO4 iv)
CH3COOH
Answer:
acid : (i) HNO3
iii) H3PO3 iv) CH3COOH
base : ii) Ba (OH)2
Lowry ŌĆō Bronsted Theory (Proton Theory)
In 1923, Lowry and Bronsted suggested a more general definition of acids
and bases. According to their concept, an acid is defined as a substance that
has a tendency to donate a proton to another substance and base is a substance
that has a tendency to accept a proton from other substance. In other words, an
acid is a proton donor and a base is a proton acceptor.
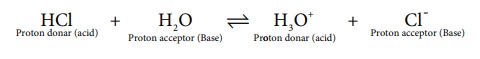
When hydrogen chloride is dissolved in water, it donates a proton to the
later. Thus, HCl behaves as an acid and H2O is base. The proton
transfer from the acid to base can be represented as
HCl +H2O Ōåö H3O+
+ Cl-
When ammonia is dissolved in water, it accepts a proton from water. In
this case, ammonia ( NH3 ) acts as a base and H2O is
acid. The reaction is represented as
H2O + NH3 Ōåö NH4+
+ OH-
Let us consider the reverse reaction in the following equilibrium
HCl + H2O Ōåö H3O+ + Cl-
Proton donar (acid) + Proton acceptor (Base) Ōåö
Proton
donar (acid) + Proton acceptor (Base)
H3O+ donates a proton to Cl- to form HCl
i.e., the products also behave as acid and base.
In general, Lowry ŌĆō Bronsted (acid ŌĆō base) reaction is represented as
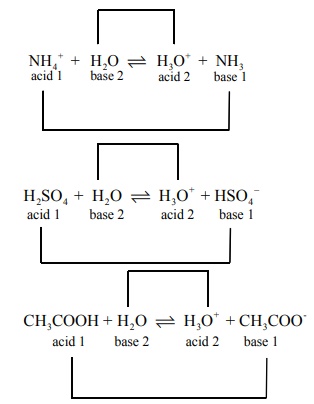
Acid1 + Base2 Ōåö Acid2 +Base1
The species that remains after the donation of a proton is a base (Base1
) and is called the conjugate base of the Bronsted acid ( Acid 1
) . In other
words, chemical species that differ only by a proton are called conjugate acid
ŌĆō base pairs.
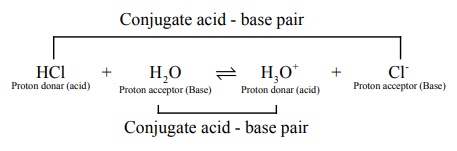
HCl and Cl- , H2O and H3O+
are two conjugate acid ŌĆō base pairs. i.e., Cl- is the conjugate base
of the acid HCl . (or) HCl is conjugate acid of Cl- . Similarly H3O+
is the conjugate acid of H2O .
Limitations of Lowry ŌĆō Bronsted theory
i. Substances like BF3 , AlCl3 etc., that do not
donate protons are known to behave as acids.
Evaluate yourself ŌĆō 2
Write a balanced equation for the dissociation of the
following in water and identify the conjugate acid ŌĆōbase pairs. i) NH4+
ii) H2SO4 iii) CH3COOH.
Answer:
Lewis concept
In 1923, Gilbert . N. Lewis proposed a more generalised concept of acids
and bases. He considered the electron pair to define a species as an acid (or)
a base. According to him, an acid is a species that accepts an electron pair
while base is a species that donates an electron pair. We call such species as
Lewis acids and bases.
A Lewis acid is a positive ion (or) an electron deficient molecule and a
Lewis base is a anion (or) neutral molecule with at least one lone pair of
electrons.
Les us consider the reaction between Boron tri fluoride and ammonia
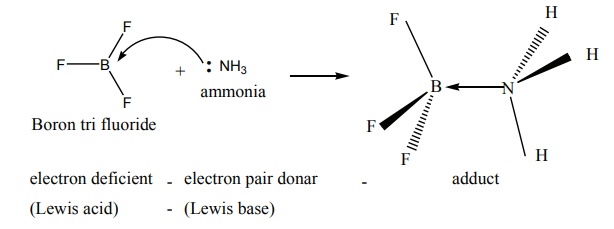
Here, boron has a vacant 2p orbital to accept the lone pair of electrons
donated by ammonia to form a new coordinate covalent bond. We have already
learnt that in coordination compounds, the Ligands act as a Lewis base and the
central metal atom or ion that accepts a pair of electrons from the ligand
behaves as a Lewis acid.
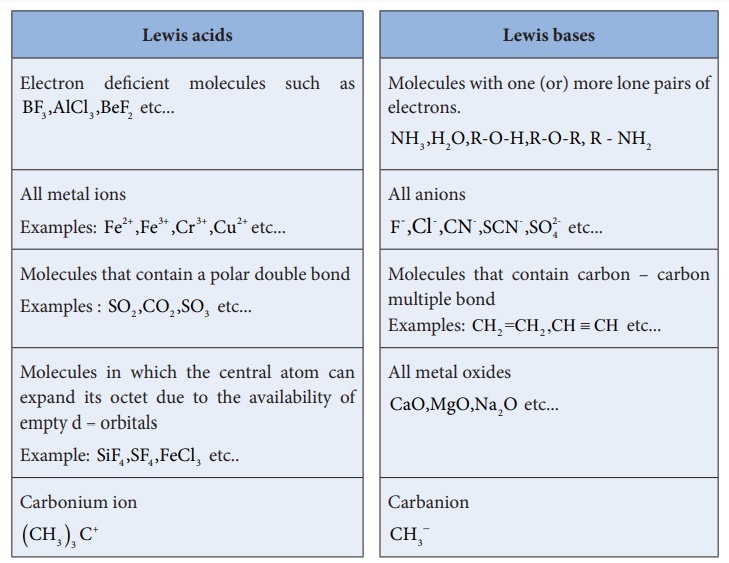
Lewis acids
ŌĆó Electron deficient molecules such as BF3 ,AlCl3
,BeF2 etc...
ŌĆó All metal ions. Examples: Fe2+ ,Fe3+ ,Cr3+
,Cu2+ etc...
ŌĆó Molecules that contain a polar double bond Examples : SO2
,CO2 ,SO3 etc...
ŌĆó Molecules in which the central atom can expand its octet due to the
availability of empty d ŌĆō orbitals. Example: SiF4 ,SF4
,FeCl3 etc..
ŌĆó Carbonium ion (CH3
)3 C+
Lewis bases
ŌĆó Molecules with one (or) more lone pairs of electrons. NH3
,H2O,R-O-H,R-O-R, R - NH2
ŌĆó All anions. F- ,Cl- ,CN- ,SCN-
,SO24- etc...
ŌĆó Molecules that contain carbon ŌĆō carbon multiple bond. Examples: CH2
=CH 2 ,CH ŌēĪ CH
etc...
ŌĆó All metal oxides. CaO,MgO,Na2O etc...
ŌĆó Carbanion. CH3ŌłÆ
Example
Identify the Lewis acid and the Lewis base in the following reactions.
Cr3+ + 6 H2 O ŌåÆ [Cr(H2O)6 ]3+
In the hydration of ion, each of six water molecules donates a pair of
electron to Cr3+ to from the hydrated cation, hexaaquachromium (III)
ion, thus, the Lewis acid is Cr3+ and the Lewis base H2O
.
Evaluate yourself ŌĆō 3
Identify the Lewis acid and the Lewis base in the
following reactions.
i. CaO+CO2 ŌåÆ CaCO3
Answer:
i) CaO - Lewis base
; CO2 ŌĆōLewisacid
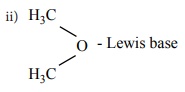
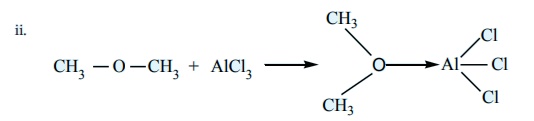
AlCl3 -
Lewis acid
Evaluate yourself ŌĆō 4
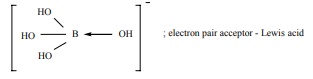
H3BO3 accepts hydroxide ion from
water as shown below
H3BO3 (aq) + H2O(l) Ōåö B(OH)4- +H+
Predict the nature of H3 BO3 using
Lewis concept
Answer:
Related Topics