Ionic Equilibrium | Chemistry - Ionisation of water | 12th Chemistry : UNIT 8 : Ionic Equilibrium
Chapter: 12th Chemistry : UNIT 8 : Ionic Equilibrium
Ionisation of water
Ionisation of water
We have learnt that when an acidic or a basic substance is dissolved in
water, depending upon its nature, it can either donate (or) accept a proton. In
addition to that the pure water itself has a little tendency to dissociate.
i.e, one water molecule donates a proton to an another water molecule. This is
known as auto ionisation of water and it is represented as below.
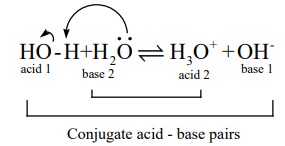
In the above ionisation, one water molecule acts as an acid while the
another water molecule acts as a base.
The dissociation constant for the above ionisation is given by the
following expression

The concentration of pure liquid water is one. i.e, [H2O]2
= 1
Kw = [H3O+
][OH- ] . ....(8.4)
Here, Kw represents the ionic product (ionic product
constant) of water
It was experimentally found that the concentration of H3O+
in pure water is 1├Ś10-7 at 25oC . Since the dissociation
of water produces equal number of H3O+ and OH-
, the concentration of OH- is also equal to 1├Ś10-7 at 25oC.
Therefore, the ionic product of water at 25oC is
Kw = [H3O]+[OH-] ......(8.4)
Kw = (1 ├Ś 10-7
)(1 ├Ś 10-7
)
= 1 ├Ś10-14.
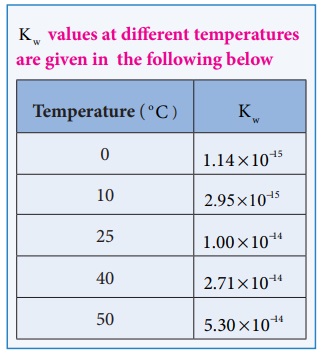
Like all equilibrium constants, Kw ŌĆēis also a constant at a
particular temperature. The dissociation of water is an endothermic reaction.
With the increase in temperature, the concentration of H 3O+
and OH- also increases, and hence the ionic product also increases.
In neutral aqueous solution like NaCl solution, the concentration of H3O+
is always equal to the concentration of OH- whereas in case of an
aqueous solution of a substance which may behave as an acid (or) a base, the
concentration of H3O+ will not be equal to [OH-].
We can understand this by considering the aqueous HCl as an example. In
addition to the auto ionisation of water, the following equilibrium due to the
dissociation of HCl can also exist.
HCl + H2O Ōåö H3O + + Cl-
In this case, in addition to the auto ionisation of water, HCl molecules
also produces H3O+ ion by donating a proton to water and
hence [H3O+]>[OH-]. It means that the
aqueous HCl solution is acidic. Similarly, in basic solution such as aqueous NH3
, NaOH etcŌĆ”.. [OHŌĆō]>[H3O+].
Example 8.1
Calculate the concentration of OH- in a fruit juice which
contains 2 ├Ś10-3
M, H3O+ ion. Identify the nature of the solution.
Given that H3O+ = 2 ├Ś10-3 M
Kw = [H3O+ ][OH - ]
Ōł┤[OH - ]= Kw /[H3O+ ] = [1 ├Ś├Ś10-14]
/ [2 x 10 3] =5 ├Ś10-12
M
2 ├Ś10-3 >> 5 ├Ś10-12
i.e., [H3O+
] >> [OH-], hence the juice is acidic in nature
Evaluate yourself ŌĆō 5
At a particular temperature, the Kw of a
neutral solution was equal to 4 ├Ś10-14. Calculate the
concentration of [H3O+ ] and [OH- ].
Answer:
Given solution is neutral
Ōł┤ [ H3O+ ] = [OH- ]
Let [H3O+
] = x ; then [OH- ] = x
Kw = [H3O+
][OH- ]
4├Ś 10-14 = x . x
x2 = 4 ├Ś 10-14
x = ŌłÜ 4 ├Ś 10-14 = 2 ├Ś10-7
Related Topics