Ionic Equilibrium | Chemistry - Choose the correct answer | 12th Chemistry : UNIT 8 : Ionic Equilibrium
Chapter: 12th Chemistry : UNIT 8 : Ionic Equilibrium
Choose the correct answer
Chemistry : Ionic Equilibrium
Choose the correct answer:
1. Concentration of the Ag+ ions in a saturated solution of Ag2 C2O4 is 2.24 ├Ś10-4mol L-1 solubility product of Ag2 C2O4 is
a) 2.42 ├Ś10-8 mol3L-3
b) 2.66 ├Ś10-12 mol3L-3
c) 4.5 ├Ś10-11 mol3L-3
d) 5.619 ├Ś 10ŌĆō12 mol3L-3
Solution:
Ag2C2O4 Ōåö 2Ag+ + C2O42-
[ Ag+ ] = 2.24 ├Ś 10-4 mol L-1
[ C2 O42- ] = { 2.24├Ś10-4 }/2 = mol L-1
= 1.12├Ś10-4 mol L-1
Ksp = [Ag+]2[C2O42-]
= (2.24├Ś10-4 mol-4 L-1 )2 (1.12├Ś10-4 mol L-1)
= 5.619├Ś10-12mol3 L-3
[Option (d)]
2. Following solutions were prepared by mixing different volumes of NaOH of HCl different concentrations.
i. 60 mL (M/10) HCl + 40mL (M/10) NaOH
ii. 55 mL (M/10) HCl + 45 mL (M/10) NaOH
iii. 75 mL (M/5) HCl + 25mL (M/5) NaOH
iv. 100 mL (M/10) HCl + 100 mL (M/10) NaOH
pH of which one of them will be equal to 1?
a) iv
b) i
c) ii
d) iii
Solution:
iii) 75 ml M/5 HCl + 25ml M/5 NaOH
No of moles of HCl = 0.2├Ś75├Ś10-3 = 15 ├Ś 10-3
No of moles of NaOH = 0.2 ├Ś 25 ├Ś 10-3 = 5 ├Ś 10-3
No of moles of HCl after mixing = 15 ├Ś 10-3 - 5 ├Ś 10-3
= 10 ├Ś 10-3
concentration of HCl = No of moles of HCl / Vol in litre
= 10 ├Ś10ŌłÆ3 / 100 ├Ś10ŌłÆ3 = 0.1M
for (iii) solution, pH of 0.1M HCl = -log10(0.1)
= 1.
[Option (d)].
3. The solubility of BaSO4 in water is 2.42 ├Ś10-3 gL-1 at 298K. The value of its solubility product ( Ksp ) will be. (Given molar mass of BaSO4 =233g mol-1 )
a) 1.08 ├Ś10-14 mol2L-2
b) 1.08 ├Ś10-12 mol 2 L-2
c) 1.08 ├Ś10-10 mol2L-2
d) 1.08 ├Ś10-8mol2 L-2
Solution:
BaSO4 Ōåö Ba2+ +SO42-
Ksp =(s) (s)
Ksp =(s)2
= ( 2.42├Ś10-3 g L-1 )2
= ( 2.42├Ś10-3 g L-1 / 233g mol-1 )
= ( 0.01038├Ś10-3 ) 2
= (1.038├Ś10-5 )2
= 1.077├Ś10-10
= 1.08├Ś10-10 mol2 L-2
[Option (c)]
4. pH of a saturated solution of Ca(OH)2 is 9. The Solubility product ( Ksp )of Ca(OH)2
a) 0.5 ├Ś10-15
b) 0.25 ├Ś10-10
c) 0.125 ├Ś10-15
d) 0.5 ├Ś10-10
Solution:
Ca(OH)2 Ōåö Ca2+ + 2OH-
Given that pH = 9
pOH = 14-9 = 5
[pOH = -log10 [OH- ]]
Ōł┤[OH- ] = 10- pOH
[OH- ]=10-5 M
Ksp =[Ca2+ ][OH- ]2
= (10-5 /2 )├Ś(10-5 )2
=0.5 ├Ś10-15
[Option (a)]
5. Conjugate base for Bronsted acids H2O and HF are
a) OH- and H2FH+ , respectively
b) H3O+ and F- , respectively
c) OH- and F- , respectively
d) H3 O+and H2F+ , respectively
Solution:
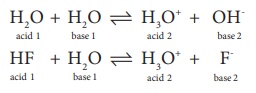
H2O + H2O Ōåö H3O+ + OH-
acid 1 + base 1 Ōåö acid 2 + base 2
HF + H2O Ōåö H3O+ + F-
acid 1 + base 1 Ōåö acid 2 + base 2
Conjugate bases are OH- and F- respectively
i.e.,[ Option (c)]
6. Which will make basic buffer?
a) 50 mL of 0.1M NaOH+25mL of 0.1M CH3COOH
b) 100 mL of 0.1M CH3COOH+100 mL of 0.1M NH4OH
c) 100 mL of 0.1M HCl+200 mL of 0.1M NH4OH
d) 100 mL of 0.1M HCl + 100 mL of 0.1M NaOH
Solution:
Basic buffer is the solution which has weak base and its salt
NH4OH(200ml) + HCl(100ml) ŌåÆNH4Cl(Salt) + H2O + NH4OH (100ml weak base)
i.e.,[ Option (c)]
7. Which of the following fluro compounds is most likely to behave as a Lewis base?
a) BF3
b) PF3
c) CF4
d) SiF4
Solution:
BF3 ŌåÆ elctron deficient ŌåÆ Lewis acid
PF3 ŌåÆ electron rich ŌåÆ lewis base
CF4 ŌåÆ neutral ŌåÆ neither lewis acid nor base
SiF4- ŌåÆ neutral ŌåÆ neither lewis acid nor base
[option (b)]
8. Which of these is not likely to act as Lewis base?
a) BF3
b) PF3
c) CO
d) FŌĆō
Solution:
BF3 ŌåÆ elctron deficient ŌåÆ Lewis acid
PF3 ŌåÆ electron rich ŌåÆ lewis base
CO ŌåÆ having lone pair of electron ŌåÆ lewis base
F- ŌåÆ unshared pair of electron ŌåÆ lewis base
[option (a)]
9. The aqueous solutions of sodium formate, anilinium chloride and potassium cyanide are respectively
a) acidic, acidic, basic
b) basic, acidic, basic
c) basic, neutral, basic
d) none of these
Solution:
HCOONa Basic in nature. + H ŌŗģOH Ōåö NaOH strong base + H-COOH weak acid
C6H5NH3Cl- + H Ōŗģ OH Ōåö H3O+Acidic +C6H5 -NH2 + Cl-
KCN basic + HŌłÆ OH Ōåö KOH strong base + HCN weak acid
basic, acidic, basic is correct.
[option (b)]
10. The percentage of pyridine (C5H5N) that forms pyridinium ion (C5 H5NH) in a 0.10M aqueous pyridine solution (Kb for C5 H5 N= 1.7 ├Ś10-9 ) is
a) 0.006%
b) 0.013%
c) 0.77%
d) 1.6%
Solution:
C5H5N + H-OH Ōåö C5H5 NH + OH-
(╬▒2C) / (1-╬▒ ) =Kb
╬▒2C =Ōł╝ Kb
╬▒ = ŌłÜ [ Kb / C ] = ŌłÜ[ 1.7 ├Ś10-9 / 0.1 ]
= ŌłÜ1.7 ├Ś 10-4
= ŌłÜ1.7 ├Ś 10ŌłÆ4 ├Ś 100
Percentage of dissociation = 1.3 ├Ś10-2 = 0.013 %
[Option (b)]
11. Equal volumes of three acid solutions of pH 1,2 and 3 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
a) 3.7 ├Ś10-2
b) 10-6
c) 0.111
d) none of these
Solution:
pH = -log10[H+]
Ōł┤[H+]=10-pH
Let the volume be x mL
V1M1 + V2M2 + V3M3 =VM
Ōł┤ x mL of 10-1M+ x mL of 10-2M + x mL of 10-3 M
= 3x mL of [H+]
Ōł┤[H+ ] = x[0.1+0.01+0.001] / 3x
= ( 0.1+ 0.01+ 0.001 ) / 3
= 0..111 / 3
= 0.037
= 3.7 ├Ś10ŌłÆ2
[Option (a)]
12. The solubility of AgCl (s) with solubility product 1.6 ├Ś10-10 in 0.1M NaCl solution would be
a) 1.26 ├Ś 10-5M
b) 1.6 ├Ś10-9M
c) 1.6 ├Ś10-11M
d) Zero
Solution:
AgCl(s) Ōåö Ag+ (aq) + Cl-(aq)
NaCl (0.1 M) ŌåÆ Na+(0.1 M) + Cl- (0.1 M)
Ksp = 1..6 ├Ś10-10
Ksp = [Ag+][Cl-]
Ksp = (s)(s+0.1)
0.1>>>s
Ōł┤ s + 0.1 = 0.1
Ōł┤ S = [ 1..6 ├Ś10-10 ] / 0.1 = 1.6 ├Ś10-9
[Option (b)]
13. If the solubility product of lead iodide is 3.2 ├Ś10-8 , its solubility will be
a) 2├Ś10-3M
b) 4 ├Ś10-4M
c) 1.6 ├Ś10-5M
d) 1.8 ├Ś10-5M
Solution:
PbI2 (s) Ōåö Pb2+ (aq) + 2I- (aq)
Ksp = (s)(2s)2
3.2 ├Ś 10-8 = 4s3
s = (3.2├Ś10-8 / 4)1/3
= (8 ├Ś 10-9 )1/3
= 2 ├Ś 10-3M
= 2 ├Ś 10-3M
[Option (a)]
14. MY and NY3 , are insoluble salts and have the same Ksp values of 6.2 ├Ś10-13 at room temperature. Which statement would be true with regard to MY and NY3?
a) The salts MY and NY3 are more soluble in 0.5M KY than in pure water
b) The addition of the salt of KY to the suspension of MY and NY3 will have no effect on their solubilityŌĆÖs
c) The molar solubilities of MY and NY3 in water are identical
d) The molar solubility of MY in water is less than that of NY3
Solution:
Addition of salt KY (having a common ion YŌĆō) decreases the solubility of MY and NY due to common ion effect.
Option (a) and (b) are wrong.
For salt MY , MY Ōåö M+ + Y-
Ksp = (s)(s)
6.2 ├Ś 10-13 = s2
Ōł┤ s= ŌłÜ(6.2 ├Ś 10-13) = 10-7
for salt NY3 ,
NY3 Ōåö N3+ + 3Y-
Ksp = (s )(3s)3
Ksp = 27s4
s = [ (6.2 ├Ś10ŌłÆ13)/27 ]1/4
s = 10-4
The molar solubility of MY in water is less than of NY3
[Option (d)]
15. What is the pH of the resulting solution when equal volumes of 0.1M NaOH and 0.01M HCl are mixed?
a) 2.0
b) 3
c) 7.0
d) 12.65
Solution:
x ml of 0.1 M NaOH + x ml of 0.01 M HCl
No of moles of NaOH = 0.1 ├Ś x ├Ś 10ŌĆō3 = 0.1x ├Ś 10-3
No of moles of HCl = 0.01 ├Ś x ├Ś 10-3 = 0.01x ├Ś 10-3
No of moles of NaOH after mixing = 0.1x ├Ś 10-3 - 0.01x ├Ś 10-3
= 0.09x ├Ś 10-3
Concentration of NaOH= [0.09x ├Ś 10ŌłÆ3 ] / [2x ├Ś 10ŌłÆ3] = 0.045
[OH-] = 0.045
POH = ŌłÆlog (4.5 ├Ś 10ŌłÆ2 )
= 2 ŌłÆlog 4.5
= 2- 0.65 = 1.35
pH = 14-1.35=12.65
Solution: Option (d)
16. The dissociation constant of a weak acid is 1 ├Ś10-3. In order to prepare a buffer solution with a pH = 4, the [Acid]/[Salt] ratio should be
a) 4:3
b) 3:4
c) 10:1
d) 1:10
Solution:
K a =1 ├Ś10-3
pH=4
[salt] / [Acid] = ?
pH = pKa +log {[Salt]/[Acid]}
4 = - log10 (1 ├Ś10-3 ) + log {[Salt]/ [Acid]}
4 = 3 + log ([Salt]/ [Acid])
1= log10 ([Salt]/[Acid])
[Salt] / [Acid] =101
i.e., [Salt] / [Acid] = 1 / 10
1:10
[Option (d)]
17. The pH of 10-5M KOH solution will be
a) 9
b) 5
c) 19
d) none of these
Solution:
KOH ŌåÆ K+ + OH-
10-5m 10-5m 10-5m
KOH (10-5m) ŌåÆ K+ (10-5m) + OH- (10-5m)
[OH-] = 10-5M.
pH= 14 - pOH
pH = 14 - ( - log [OH-] )
= 14 + log [OH- ]
= 14 + log10ŌłÆ5
= 14ŌłÆ5
= 9.
[Option (a)]
18. H2PO4- the conjugate base of
a) PO43ŌłÆ
b) P2O5
c) H3PO4
d) HPO42-
Solution:
H3PO4 +H ŌłÆ OH Ōåö H3O+ + H2PO4-
acid 1 + base 1 Ōåö acid 2 + base 2
Ōł┤H2PO4ŌłÆ is the conjugate base of H3PO4
[Option (c)]
19. Which of the following can act as Lowry ŌĆō Bronsted acid as well as base?
a) HCl
b) SO42ŌłÆ
c) HPO42ŌłÆ
d) Br-
Solution:
HPO42ŌłÆ can have the ability to accept a proton to form H2POŌłÆ4.
It can also have the ability to donate a proton to form PO4-3
[Option (c)]
20. The pH of an aqueous solution is Zero. The solution is
a) slightly acidic
b) strongly acidic
c) neutral
d) basic
Solution:
pH = -log10[H+ ]
Ōł┤[H+ ]=10-pH
=100 =1
[H+] = 1M
The solution is strongly acidic
[Option (b)]
21. The hydrogen ion concentration of a buffer solution consisting of a weak acid and its salts is given by
a) [H+] = Ka ([acid][salt])
b) [H+] = Ka[salt]
c) [H+] = Ka[acid]
d) [H+] = Ka ([salt][acid])
Solution:
According to Henderson equation
pH = pKa + log ([salt]/[acid])
ie. - log [H+ ] = - log Ka +log ([salt] / [acid])
-log[H+] = log ([salt]/ [acid]) ├Ś (1/Ka)
log (1/[H+]) = log ([salt]/ [acid]) ├Ś (1/Ka)
Ōł┤[H+] = Ka {[acid] / [salt] }
[option (a)]
22. Which of the following relation is correct for degree of hydrolysis of ammonium acetate?
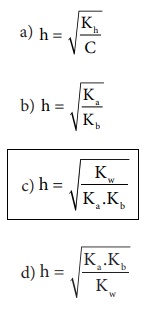
[Option (c)]
Solution:
h= ŌłÜ{ (Kh) / (Ka .Kb) }
[Option (c)]
23. Dissociation constant of NH4OH is 1.8 ├Ś10-5 the hydrolysis constant of NH4Cl would be
a) 1.8 ├Ś10-19
b) 5.55 ├Ś10-10
c) 5.55 ├Ś10-5
d) 1.80 ├Ś10-5
Solution:
Kh = Kw/Kb = 1├Ś10-14 / 1.8├Ś10-5
= 0.55├Ś10-9
= 5.5├Ś10-10
[option (b)]
PTA Question
Oneword:
1. In which of the following
cases, the sparingly soluble salt solution is unsaturated?
a)
Ionic product > solubility product (Ksp)
b) Ionic product < solubility
product (Ksp)
c)
Ionic product = solubility product (Ksp)
d)
Both (a) and (b)
Answer: b)
2. Which of the following salts
do not undergo salt hydrolysis?
a)
Sodium acetate
b)
Ammonium acetate
c)
Ammonium chloride
d) Sodium nitrate
Answer: d)
3. The relationship between the
solubility product (Ksp) and molar solubility (S) for Ag2(CrO4)
is
a)
Ksp = s3
b)
Ksp = s2
c) Ksp = 4s3
d)
Ksp = 3s2
Answer: c)
4. The aqueous solution sodium
acetate, ammonium chloride, sodium nitrate are respectively
a)
Neutral, acidic, basic
b)
acidic, basic, neutral
c)
basic, acidic, neutral
d) basic, acidic, basic
Answer: d)
Related Topics