Ionic Equilibrium | Chemistry - Salt Hydrolysis | 12th Chemistry : UNIT 8 : Ionic Equilibrium
Chapter: 12th Chemistry : UNIT 8 : Ionic Equilibrium
Salt Hydrolysis
Salt Hydrolysis
When an acid reacts with a base, a salt and water are formed and the
reaction is called neutralization. Salts completely dissociate in aqueous
solutions to give their constituent ions. The ions so produced are hydrated in
water. In certain cases, the cation, anion or both react with water and the
reaction is called salt hydrolysis. Hence, salt hydrolysis is the reverse of
neutralization reaction.
Salts of strong acid and a strong base
Let us consider the reaction between NaOH and nitric acid to give sodium
nitrate and water.
NaOH(aq)+HNO3 (aq) ŌåÆ NaNO3 (aq) + H2O(l)
The salt NaNO3 completely dissociates in water to produce Na+
and NO3ŌłÆ ions.
NaNO3 (aq) ŌåÆ Na+
(aq) + NO3- (aq)
Water dissociates to a small extent as
H2O(l) Ōåö H+ (aq)
+ OH- (aq)
Since [H + ]=[OH - ], water is neutral
NO3- ion is the conjugate base of the strong acid
HNO3 and hence it has no tendency to react with H+ .
Similarly, Na+ is the conjugate acid of the strong base NaOH
and it has no tendency to react with OH- .
It means that there is no hydrolysis. In such cases [H+] = [OH-]
pH is maintained and, therefore, the solution is neutral.
Hydrolysis of Salt of strong base and weak acid (Anionic Hydrolysis).
Let us consider the reactions between sodium hydroxide and acetic acid
to give sodium acetate and water.
NaOH (aq) + CH3 COOH(aq) Ōåö CH3COONa(aq)
+ H2O(l)
In aqueous solution, CH3COONa is completely dissociated as
below
CH3 COONa (aq) ŌåÆ CH3COO- (aq)+Na+
(aq)
CH3COO- is a conjugate base of the weak acid CH3COOH
and it has a tendency to react with H+ from water to produce
unionised acid .
There is no such tendency for Na+ to react with OH- .
CH3COO-
(aq) + H 2 O(l) Ōåö CH3COOH
(aq) + OH- (aq) and therefore [OH- ] > [H+
] , in such cases, the solution is basic due to hydrolysis and the pH is greater
than 7.
Let us find a relation between the equilibrium constant for the
hydrolysis reaction (hydrolysis constant) and the dissociation constant of the
acid.
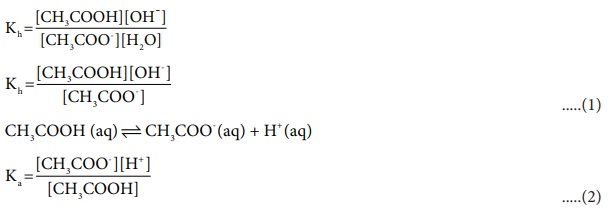
(1) ├Ś (2)
ŌćÆ Kh .Ka =[H+ ][OH-
]
we know that [H+ ][OH- ]=Kw
Kh .Ka =Kw
Kh value in terms of degree of hydrolysis (h) and the
concentration of salt (C) for the equilibrium can be obtained as in the case of
ostwald's dilution law. Kh = h2C. and i.e [OH -
] = ŌłÜ (Kh .C)
pH of salt solution in terms of Ka and the concentration of the electrolyte.
pH + pOH = 14
pH = 14 - p OH = 14 - {-log [OH- ]}
= 14 + log [OH- ]
Ōł┤pH = 14 + log (KhC)1/2
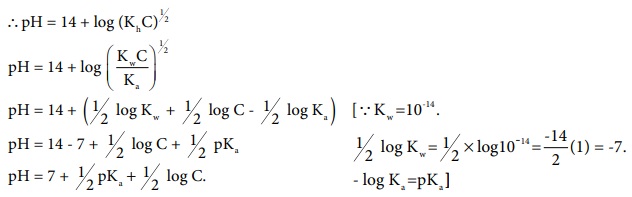
Hydrolysis of salt of strong acid and weak base (Cationic Hydrolysis)
Let us consider the reactions between a strong acid, HCl, and a weak
base, NH4OH, to produce a salt, NH4Cl , and water
HCl (aq) + NH4OH (aq) Ōåö NH4Cl(aq)
+ H2O(l)
NH4Cl(aq) ŌåÆ NH4+
+Cl- (aq)
NH4+ is a strong conjugate acid of the weak base NH4OHand it has
a tendency to react with OH- from water to produce unionised NH4OH
shown below.
NH4+ (aq) + H2O(l) Ōåö NH4OH (aq) + H+ (aq)
There is no such tendency shown by Cl- and therefore [H+]
> [OH-
]; the solution is acidic and the pH is less than 7.
As discussed in the salt hydrolysis of strong base and weak acid. In
this case also, we can establish a relationship between the Kh and Kb
as
Kh .Kb = Kw
Let us calculate the Kh value in terms of degree of
hydrolysis (h) and the concentration of salt
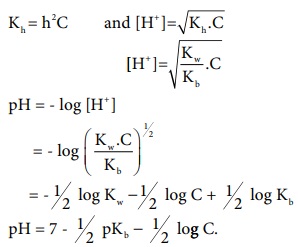
= 1/2 log Kw ŌĆō 1/2 log
C + 1/2 log Kb
pH = 7 ŌĆō 1/2 pKb ŌĆō 1/2
log C.
Hydrolysis of Salt of weak acid and weak base (Anionic & Cationic Hydrolysis).
Let us consider the hydrolysis of ammonium acetate.
CH3 COONH 4 (aq) ŌåÆ CH 3 COO - (aq) + NH4+
(aq)
In this case, both the cation (NH4+ ) and
anion (CH3COOŌłÆ) have the tendency to react with water
CH3COOŌłÆ + H2O Ōåö CH3COOH + OHŌłÆ
NH4+ + H2O Ōåö NH4OH + H+
The nature of the solution depends on the strength of acid (or) base
i.e, if Ka >Kb ; then the solution is acidic and pH
< 7, if Ka < Kb ; then the solution is basic and pH
> 7, if Ka =Kb ; then the solution is neutral.
The relation between the dissociation constant (Ka ,Kb
) and the hydrolysis constant is given by the following expression.
Ka.Kb.Kh = Kw
pH of the solution
pH of the solution can be calculated using the following
expression, pH = 7 + 1/2 pKa ŌłÆ 1/2 pKb
.
Example 8.8
Calculate i) degree of hydrolysis, ii) the hydrolysis constant and iii)
pH of 0.1M CH3COONa solution ( pKa for CH3COOH
is 4.74).
Solution (a) CH3COONa is a salt of weak acid (CH 3COOH) and a
strong base (NaOH).
Hence, the solutions is alkaline due to hydrolysis.
CH3COO - (aq) + H2O(aq)
Ōåö CH3COOH (aq) + OH-(aq)
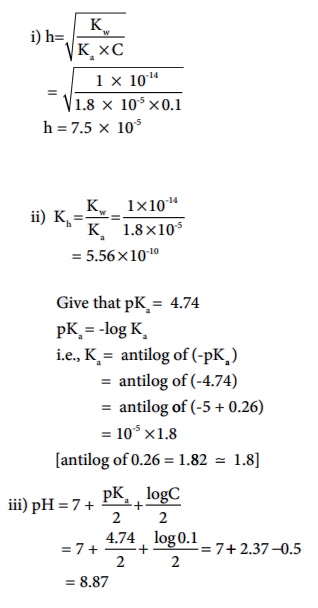
Evaluate yourself ŌĆō 10
Calculate the i) hydrolysis constant, ii) degree of hydrolysis
and iii) pH of 0.05M sodium carbonate solution ( pKa for HCO3ŌłÆ is 10.26).
Answer:
Sodium carbonate is
a salt of weak acid, H2CO3 and a strong base, NaOH, and
hence the solution is alkaline due to hydrolysis.
Na2CO3
(aq) ŌåÆ 2Na+ (aq) + CO32- (aq)
CO32-
(aq)+ H2O (l) Ōåö HCO3- +OH-
i) h= ŌłÜ{ (Kw) / (Ka ├Ś C) }
= ŌłÜ ( 1 ├Ś 10-14 / 5.5 ├Ś 10-11 ├Ś 0.05 )
h = 6.03 ├Ś 10-2
Given that pKa
=10.26
pKa =
-log Ka
i.e., Ka
=antilog of (-pKa )
= antilog of (-10.26)
= antilog of (-11 + 0.74)
= 10-11 ├Ś 5.5
[antilog of 0.74 = 5.49 Ōēł 5.5]
ii) Kh =
Kw/Ka = ( 1├Ś10-14 / 5.5 ├Ś10-11 )
= 1.8 ├Ś10-4
iii) pH = 7 + pKa/2
+ logC/2
= 7 + (10.26/2) + (log 0.05)/2 = 7 + 5.13
ŌłÆ0.65
= 11.48
Related Topics