Chapter: Biochemistry: Viruses, Cancer, and Immunology
The Immune System: Molecular Aspects
The Immune System: Molecular
Aspects
What are antibodies?
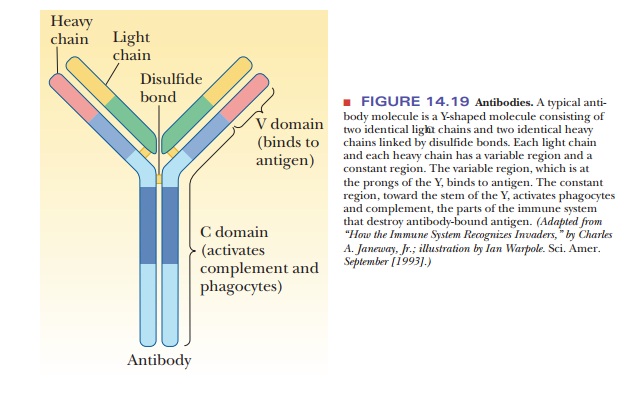
Antibodies are Y-shaped molecules consisting of two identical heavy
chains and two identical light chains held together by disulfide bonds (Figure
14.19). They are glycoproteins, with oligosaccharides linked to their heavy
chains. There are different classes of antibodies, based on differences in the
heavy chains. In some of these classes, heavy chains are linked to form dimers,
trimers, or pentamers. Each light chain and each heavy chain has a constant
region and a variable region. The variable region (also called the V domain) is found at the prongs of the
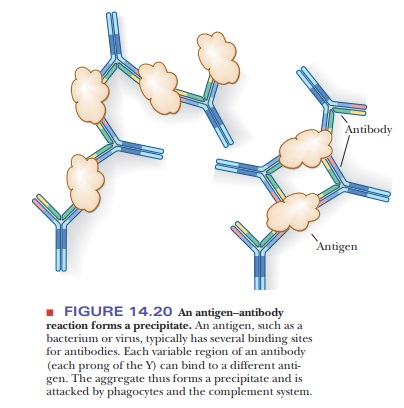
Y and is the part of the antibody that binds to the antigen (Figure 14.20). The
binding sites for the antibody on the antigen are called epitopes. Most antigens have several such binding sites, so that
the immune system has several possible avenues of attack for naturally
occurring antigens. Each antibody can bind to two antigens, and each antigen
usually has multiple binding sites for antibodies, giving rise to a precipitate
that is the basis of experimental methods for immunological research. The
constant region (the C domain) is
located at the hinge and the stem of the Y; this part of the antibody is
recognized by phagocytes and by the complement system (the portion of the
immune system that destroys antibody-bound antigen).
How does the body produce so many highly diverse antibodies to respond to essentially any possible antigen? The number of possible antibodies is virtually unlimited, as is the number of words in the English language. In a language, the letters of the alphabet can be arranged in countless ways to give a variety of words, and the same possibility for enormous numbers of rearrangements exists with the gene segments that code for portions of antibody chains. Antibody genes are inherited as small fragments that join to form a complete gene in individual B cells as they develop (Figure 14.21). When gene segments are joined, the enzymes that catalyze the process add random DNA bases to the ends of segments being spliced, allowing for the wide variety observed experimentally.
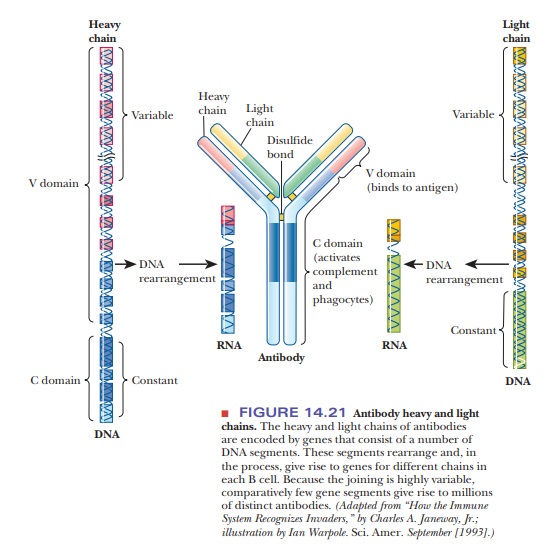
This rearrangement process takes place in the genes
for both the light and the heavy chains. In addition to these factors, B
lymphocytes have a particularly high rate of somatic muta-tion, in which
changes in the base sequence of DNA occur as the cell develops. Changes outside
the germ cells apply only to the organism in which they take place and are not
passed on to succeeding generations.
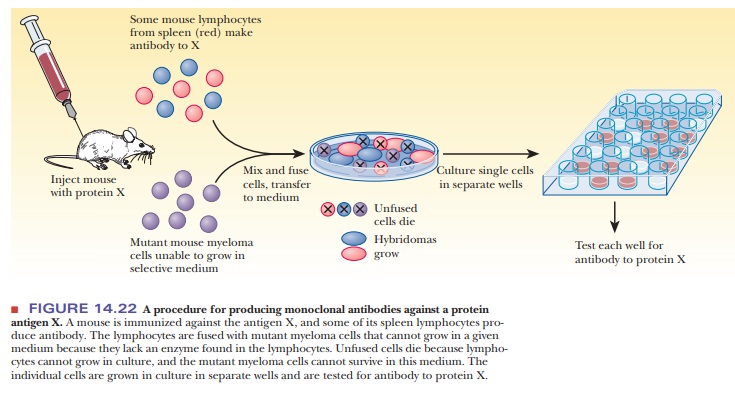
Each B cell (and each progeny plasma cell) produces only one kind
of anti-body. In principle, each such cell should be a source of a supply of
homoge-neous antibody by cloning. This is not possible in practice because
lymphocytes do not grow continuously in culture. In the late 1970s, Georges
Köhler and César Milstein developed a method to circumvent this problem, a feat
for which they received the Nobel Prize in physiology in 1984. The technique
requires fusing lymphocytes that make the desired antibody with mouse myeloma
cells. The resulting hybridoma
(hybrid myeloma), like all cancer cells, can be cloned in culture (Figure
14.22) and produces the desired antibody. Because the clones are the progeny of
a single cell, they produce homogeneous monoclo-nal
antibodies. In this way, it is possible to produce antibodies to almost
anyantigen in quantity. Monoclonal antibodies can be used to assay for
biological substances that can act as antigens. A striking example of their
usefulness is in testing blood for the presence of HIV; this procedure has
become routine to protect the public blood supply.
Related Topics