Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Critical Care
Reversible Azotemia Versus AKI
REVERSIBLE AZOTEMIA VERSUS AKI
It is important to differentiate prerenal and
postrenal azotemia from renal azotemia. Exclusion of postre-nal azotemia
requires physical diagnosis and imag-ing, whereas exclusion of prerenal
azotemia depends on the response to treatments aimed at improving renal
perfusion. Diagnosis and treatment may be facilitated by analysis of urine (see
Table 57–8); uri-nary composition in postrenal azotemia is variable and depends
on the duration and severity of obstruc-tion. In prerenal azotemia, tubular
concentrating ability is preserved and reflected by a low urinary sodium
concentration and high urine/serum creati-nine ratio. Calculation of the
fractional excretion of filtered sodium (F ENa+) may also be extremely use-ful in the setting of oliguria:
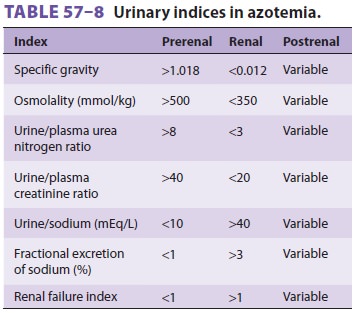
FENa+ is less than 1% in oliguric patients with prerenal azotemia but
typically exceeds 3% in patients with oliguric AKI. Values of 1–3% may be
present in patients with nonoliguric AKI. The renal failure index, which is the
urinary sodium concen-tration divided by the urine/plasma creatinine ratio, is
a sensitive index for diagnosing kidney failure. The use of diuretics increases
urinary sodium excre-tion and invalidates indices that rely on urinary sodium
concentration as a measure of tubular func-tion. Moreover, intrinsic kidney
diseases that pri-marily affect renal vasculature or glomeruli may not affect
tubular function and therefore are associated with indices that are similar to
prerenal azotemia. Measurement of a 3-h creatinine clearance can esti-mate the
residual glomerular filtration rate but may underestimate the degree of renal
impairment if the serum creatinine concentration is still rising.
Etiology of AKI
Causes of AKI are listed in Table
57–9. Up to 50% of cases follow major trauma or
surgery; in the majority of instances, ischemia and nephrotoxins are
responsible. AKI associated with ischemia is often termed acute tubular necrosis. Postischemic acute tubular necrosis follows
certain surgical pro-cedures more frequently than others: open abdomi-nal
aortic aneurysm resection, cardiac surgery with cardiopulmonary bypass, and
operations to relieve obstructive jaundice. Aminoglycosides, ampho-tericin B,
radiographic contrast dyes, cyclosporine, and cisplatin are the most commonly
implicated exogenous nephrotoxins. Amphotericin B, con-trast dyes, and
cyclosporine also appear to produce direct intrarenal vasoconstriction. Hemoglobin
and myoglobin are potent nephrotoxins when they are released during
intravascular hemolysis and rhabdomyolysis, respectively. Cyclooxygenase
inhibitors, particularly nonsteroidal antiinflam-matory drugs, may play an
important role in at least some patients. Inhibition of prostaglandin
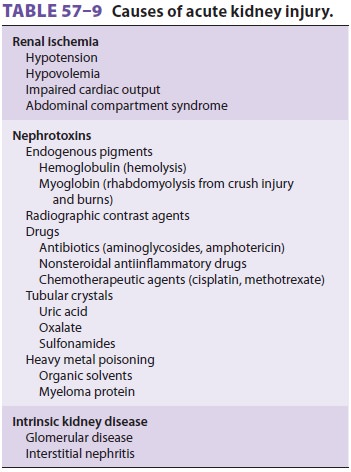
synthesis by the latter group of agents decreases prostaglandin-mediated
renal vasodilation, allow-ing unopposed renal vasoconstriction. Other fac-tors
predisposing to AKI include preexisting renal impairment, advanced age,
atherosclerotic vascular disease, diabetes, and dehydration.
Pathogenesis of AKI
The sensitivity of the kidneys to injury may be explained by their very
high metabolic rate and ability to concentrate potentially toxic substances.
The pathogenesis of AKI is complex and probably has both a vascular endothelial
and a renal epithe-lial (tubular) basis. Inadequate oxygen deliver to the
kidney is the likely triggering event, leading to afferent arteriolar constriction,
decreased glo-merular permeability, increased vascular perme-ability, altered
coagulation, inflammation, leukocyte activation, direct epithelial cell injury,
and tubular obstruction from intraluminal debris or edema. All can decrease
glomerular filtration. A backleak of filtered solutes through damaged portions
of renal tubules may allow reabsorption of creatinine, urea, and other
nitrogenous wastes.
Oliguric versus Nonoliguric AKI
AKI is often classified as oliguric (urinary
volume <400 mL/d), anuric
(urinary volume <100 mL/d), or nonoliguric (urinary volume >400 mL/d). Nonoli-guric AKI accounts for up
to 50% of cases. Urinary sodium concentrations in patients with nonoligu-ric
AKI are typically lower than those in oliguric patients. In some studies,
nonoliguric patients also appear to have a lower complication rate and to
require shorter hospitalizations. In another study of AKI patients who required
dialysis, nonoliguric AKI patients had a delayed initiation of dialysis, a
longer hospital stay, and an increased likelihood of death. It was speculated
that it might be possible to convert oliguric AKI into nonoliguric AKI by
administering mannitol, furosemide, “renal” doses of dopamine (1–2 mcg/kg/min),
or fenoldopam. Theoretically, the resulting increase in urinary output might be
therapeutic by preventing tubular obstruction. How-ever, recent studies have
found increased mortality in patients with AKI who received diuretics, and a
meta-analysis showed no improvement in mortality or decrease in need for
dialysis; therefore diuretics should not be routinely administered in AKI.
Treatment of AKI
AKI accounts for approximately 15% of ICU
admis-sions. Despite advances in critical care medicine, the mortality rate for
AKI remains approximately 50% and management is primarily supportive.
Diuret-ics continue to be useful for conventional medical indications (eg,
pulmonary edema or rhabdomy-olysis). AKI due to glomerulonephritis or
vasculitis may respond to glucocorticoids. Standard treatment for oliguric and
anuric patients includes restric-tion of fluid, sodium, potassium, and
phospho-rus. Daily weight measurements help guide fluid therapy. Sodium and
potassium intake is limited to 1 mEq/kg/d. Hyponatremia can be treated with
water restriction. Hyperkalemia may require admin-istration of an ion-exchange
resin (sodium polysty-rene), glucose and insulin, calcium gluconate, or sodium
bicarbonate. Sodium bicarbonate therapy may also be necessary for metabolic
acidosis when the serum bicarbonate level decreases to less than 15 mEq/L.
Hyperphosphatemia requires dietary phosphate restriction and phosphate binders
such as sevelamer, aluminum hydroxide, calcium carbon-ate, calcium acetate. The
dosages of renally excreted drugs should be adjusted to the estimated
glomeru-lar filtration rate or measured creatinine clearance to prevent
accumulation.
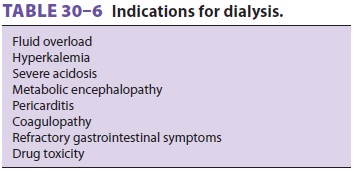
Renal replacement therapy may be employed to treat or prevent uremic
complications (see Table 30–6). A double-lumen catheter placed in the internal
jugular, subclavian, or femoral vein is usu-ally used. The high morbidity and
mortality rates associated with AKI would seem to argue for early dialysis, but
supporting studies are controversial. Dialysis does not appear to hasten
recovery but C may in fact aggravate kidney injury if hypotension occurs or too
much fluid is removed.
Because of concern that intermittent
hemodialy-sis associated with hypotension may perpetuate renal injury, continuous renal replacement therapy
(CRRT; continuous venovenous hemofiltration or continu-ous venovenous
hemodialysis, which removes fluid and solutes at a slow controlled rate) has
been used in critically ill patients with uremic AKI who do not tolerate the
hemodynamic effects of intermittent “standard” hemodialysis. The main problem
asso-ciated with CRRT is the expense, as the membrane is prone to clot
formation and, therefore, must be periodically replaced. Despite this
limitation, many experts believe CRRT is the best way to manage uremic ICU
patients with AKI. The indications for CRRT are being expanded from oliguria
and uremia to metabolic acidosis, fluid overload, and hyperkale-mia.
Nevertheless, recent clinical trials have failed to show benefit of continuous
technique over intermit-tent hemodialysis in these critically ill patients.
The nutritional management of AKI with ure-mia continues to evolve, and
there is now consensus among nephrologists, intensivists, and nutritionists
that nutrition should be provided, and 1.0–1.5 g/kg/d of protein can be given,
particularly for patients on CRRT.
Related Topics