Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Critical Care
Ambient Oxygen Therapy Equipment: Fixed-Performance (High-Flow) Equipment
Fixed-Performance
(High-Flow) Equipment
Anesthesia Bag or Bag-Mask-Valve Systems
The basic design follows that of the
nonrebreathing reservoir mask but with more “capable” components.
Self-inflating bags consist of a roughly 1.5 L bladder, usually with an oxygen
inlet reservoir. Anesthesia bags are 1-, 2-, or 3-L non–self-inflating
reservoirs with a tailpiece gas inlet. Masks are designed to pro-vide a
comfortable leak-free seal for manual ventila-tion. The inspiratory/expiratory
valve systems may vary. The flow to the reservoir should be kept high so that
the bags do not deflate substantially. When using an anesthesia bag, operators
may frequently have to adjust the oxygen flow and exhaust valve to respond to
changing breathing patterns or demands, particularly when maintaining a
complete seal between the mask and face is difficult.
The most common systems for disposable and permanent self-inflating
resuscitation bags use a unidirectional gas flow. Although these devices offer
the potential for a constant FIO2 greater than 0.9, tailpiece
inlet valves will not open for a spon-taneously breathing patient. Opening the
valves requires negative pressure bag recoil after compres-sion. If this
situation is not recognized, clinicians might be misled into thinking the
patient is receiv-ing a specific concentration of oxygen when this is not the
case.
There are limits to the ability of each system to maintain its
fixed-performance characteristics. Delivered FIO2 can approach 1.0 with either
anesthe-sia or self-inflating bags. Spontaneously breathing patients are
allowed to breathe only the contents of the system if the mask seal is tight
and the reservoir is adequately maintained.
Failure to maintain an adequate oxygen sup-ply in the reservoir and
inlet flow is a concern. The spring-loaded valve of anesthesia bags must be adjusted
to prevent overdistention of the bag. Self-inflating bags look the same whether
or not oxygen flow to the unit is adequate, and they will entrain room air into
the bag, thus lowering the delivered FIO2.
Air-Entraining Venturi Masks
The gas delivery approach with air-entraining masks is different than
with an oxygen reservoir. The goal is to create an open system with high f low
about the nose and mouth, with a fixed FIO2. Oxygen is directed by
small-bore tubing to a mixing jet; the final oxygen concentration depends on
the ratio of air drawn in through entrainment ports. Manu-facturers have
developed both fixed and adjustable entrainment selections over an FIO2 range.
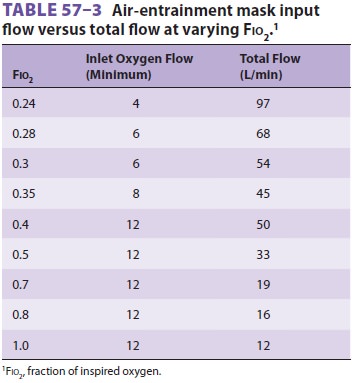
Most provide instructions for the operator to set a mini-mum flow of oxygen. Table
57–3 identifies total flow at various inlet flows and FIO2.
Despite the high-flow concept, FIO2 can vary up to 6% from the anticipated
setting. The air-entraining masks are a logical choice for patients who require
greater FIO2
than can be provided by devices such as the nasal cannula. Patients with COPD
who tend to hypoventilate with a moderate FIO2 are candidates for the Venturi mask. Clinicians providing oxygen
therapy with Venturi masks should be aware of the previously mentioned problems
involving the mask itself. FIO2 can increase if the air entrainment ports are obstructed by the
patient’s hands, bed sheets, or water condensate. Clinicians should encourage
the
patient and caregivers to keep the mask on the face continuously.
Interruption of oxygen is a serious problem in unstable patients with hypoxemia
and or hypercarbia.
Direct analysis of the FIO2 during air-entrain-ment mask breathing is
difficult to perform accu-rately. Arterial blood gas analysis and the patient’s
respiratory rate should guide clinicians as to whether the patient’s demands
are being met by the mask’s flow. If that occurs, then inlet oxygen flows may
need to be increased or an alternate device selected.
Air-Entraining Nebulizers
Large-volume, high-output or “all-purpose”
nebu-lizers have been used in respiratory care for many years to provide mist
therapy with some control of the FIO 2. These units are commonly placed on patients following extubation for
their aerosol-pro-ducing properties. Like the air-entraining masks, nebulizers
use a pneumatic jet and an adjustable orifice to vary entrained air for varying
FIO2
lev-els. Many commercial devices have an inlet orifice diameter that maximally
allows only 15 L/min when the source pressure is 50 psi. This means that on the
100% setting (no air entrainment) output flow is only 15 L/min. Only patients
breathing at slow rates and small VT will receive 100% oxygen. This problem has
been addressed by the development of high-flow, high-FIO2 nebulizers. For more common applications
that use an FIO2
of 0.3–0.5, room air is entrained, reducing the FIO2 and increasing the total flow output to
40–50 L/min.
Knowledge of the air/oxygen ratio and the
input flow rate of oxygen allows the total outflow to be calculated. Nebulizer
systems can be applied to the patient with many different devices, including
aerosol, tracheostomy dome/collar, face tent, and Τ-piece adapter. These
appliances can all be attached via large-bore tubing to the nebulizer. This
open system freely vents inspiratory and expiratory gases around the patient’s
face or out a distal port of a Τ-piece adapter. Unfortunately, the lack of any
valves allows patients to secondarily entrain room air. It is common practice
to use either a reservoir bag before the Τ-piece or a reservoir tube on the
distal side of the Τ-piece to provide a larger volume of gas than that coming from the nebulizer. A typical
concern of those applying air-entrainment aerosol therapy with controlled
oxygen concentration is whether the system will provide adequate flow.
Clinicians should observe the mist like a tracer to determine adequacy of flow.
When a Τ-piece is used and the visible mist (exiting the distal port)
disappears during inspira-tion, the flow is inadequate.
Another concern in clinical practice is that excess water in the tubing
collects and can obstruct gas flow completely or can offer increased resistance
to flow. The latter may increase the FIO2 above the desired setting.
Other complications include bron-chospasm or laryngospasm in some patients as a
consequence of airway irritation from sterile water droplets (condensate of the
aerosol). In such cir-cumstances, a heated (nonaerosol) humidification system
should be substituted.
High-Flow Air–Oxygen Systems
Dual air–oxygen flowmeters and air–oxygen
blend-ers are commonly used for oxygen administration as well as freestanding
continuous positive airway pres-sure (CPAP) and “add-on” ventilator systems.
These systems differ from the air-entraining nebulizers, as their total output
flows do not diminish at FIO2 greater than 0.4. With these high-flow systems, the total flow to the
patient and FIO2
can be set indepen-dently to meet patient needs. This can be done using a large
reservoir bag or constant flows in the range of 50 to more than 100 L/min.
Clinicians can use a variety of appliances with these systems, including
aerosol masks, face tents, or well-fitted nonrebreath-ing system masks with
air–oxygen blenders. Face-sealing mask systems can also be constructed with a
reservoir bag and a safety valve to allow breathing if the blender fails. The
high flows of gas require use of heated humidifiers of the type commonly used
on mechanical ventilators. Humidification offers an advantage for patients with
reactive airways. Because of the high flows, such systems are used to apply
CPAP or BIPAP for spontaneously breathing patients.
Oxygen Hoods
Although many of the devices previously described have pediatric-sized
options, many infants and neonates will not tolerate facial appliances. Oxy-gen
hoods cover only the head, allowing access to the child’s lower body while
still permitting use of a standard incubator or radiant warmer. The hood is
ideal for relatively short-term oxygen therapy for newborns and inactive
infants. However, for mobile infants requiring longer term therapy, the nasal
cannula, face mask, or full-bed enclosure allow for greater mobility.
Normally, oxygen and air are premixed by an
air–oxygen blender and passed through a heated humidifier. Nebulizers should be
avoided. Most pneumatic jet-type nebulizers create noise levels (>65 dB) that may cause
newborn hearing loss, and cold gas can induce an increase in oxygen
consump-tion. Hoods come in different sizes. Some are sim-ple Plexiglas boxes;
others have elaborate systems for sealing the neck opening. There is no attempt
to completely seal the system, as a constant flow of gas is needed to remove
carbon dioxide (minimum flow >7 L/min). Hood inlet flows of 10–15 L/min are adequate for a majority of
patients.
Helium–Oxygen Therapy
Helium–oxygen (heliox) mixtures have a notable, yet limited clinical
role. In addition to its uses in industry and deep-sea diving, heliox has a
number of medical applications. Helium is premixed with oxygen in several
standard blends. The most popu-lar mixture is 79%/21% helium–oxygen, which has
a density that is 40% that of pure oxygen. Helium– oxygen mixtures are
available in large-sized com-pressed gas cylinders.
In anesthetic practice, pressures needed to
ven-tilate patients with small-diameter tracheal tubes can be substantially
reduced when the 79%/21% mixture is used. Heliox can provide patients with
upper airway–obstructing lesions (eg, subglottic edema, foreign bodies, and
tracheal tumors) with relief from acute distress until more definitive care can
be delivered. The evidence is less convincing in treating lower airway
obstruction from COPD or acute asthma. Helium mixtures may also be used as the
driving gas for small-volume nebulizers in bron-chodilator therapy for asthma.
However, with heliox, the nebulizer flow needs to be increased to 11 L/min
versus the usual 6–8 L/min with oxygen. Patients’ work of breathing can be
reduced with heliox as compared to a conventional oxygen/nitrogen gas mixture.
Hyperbaric Oxygen
Hyperbaric oxygen therapy uses a pressurized chamber to expose the
patient to oxygen tensions exceeding ambient barometric pressure (at sea level
the ambient pressure is 760 mm Hg). With a one-person hyperbaric chamber, 100%
oxygen is usually used to pressurize the chamber. Larger chambers allow for the
simultaneous treatment of multiple patients and for the presence of medical
personnel in the chamber with patients. Multi-place chambers use air to
pressurize the chamber, whereas patients receive 100% oxygen by mask, hood, or
tracheal tube. Common indications for hyperbaric oxygen include decompression
sickness (the “bends”), certain forms of gas embolism, gas gangrene, carbon
monoxide poisoning, and treat-ment of certain wounds.
Related Topics