Equation, Main features - Quantum mechanical model of atom - Schrodinger Equation | 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Quantum mechanical model of atom - Schrodinger Equation
Quantum
mechanical model of atom ŌĆō Schrodinger Equation:
The motion of objects that we come across in our daily
life can be well described using classical mechanics which is based on the
NewtonŌĆÖs laws of motion. In classical mechanics the physical state of the
particle is defined by its position and momentum. If we know both these
properties, we can predict the future state of the system based on the force
acting on it using classical mechanics. However, according to HeisenbergŌĆÖs
uncertainty principle both these properties cannot be measured simultaneously
with absolute accuracy for a microscopic particle such as an electron. The
classical mechanics does not consider the dual nature of the matter which is
significant for microscopic particles. As a consequence, it fails to explain
the motion of microscopic particles. Based on the Heisenberg's principle and the
dual nature of the microscopic particles, a new mechanics called quantum
mechanics was developed.
Erwin Schr├Čdinger expressed the wave nature of electron in
terms of a differential equation. This equation determines the change of wave
function in space depending on the field of force in which the electron moves.
The time independent Schr├Čdinger equation can be expressed as,
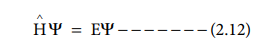
Where H Ōł¦ is called Hamiltonian operator,
╬© is the wave function and is a funciton of position
co-ordinates of the particle and is denoted as ╬©(x, y,z) E is the energy of the
system
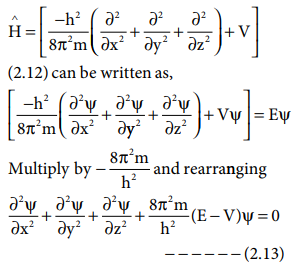
The above schrÛdinger wave equation does not contain time
as a variable and is referred to as time independent Schr├Čdinger wave equation.
This equation can be solved only for certain values of E, the total energy.
i.e. the energy of the system is quantised. The permitted total energy values
are called eigen values and corresponding wave functions represent the atomic
orbitals.
Main features of the quantum mechanical model of atom
1. The energy of electrons in atoms is quantised
2. The existence of quantized electronic energy levels is
a direct result of the wave like properties of electrons. The solutions of
Schr├Čdinger wave equation gives the allowed energy levels (orbits).
3. According to Heisenberg uncertainty principle, the exact
position and momentum of an electron can not be determined with absolute
accuracy. As a consequence, quantum mechanics introduced the concept of
orbital. Orbital is a three dimensional space in which the probability of
finding the electron is maximum.
4. The solution of SchrÛdinger wave equation for the
allowed energies of an atom gives the wave function Žł, which represents an
atomic orbital. The wave nature of electron present in an orbital can be well
defined by the wave function Žł.
5. The wave function Žł itself has no physical meaning.
However, the probability of finding the electron in a small volume dxdydz
around a point (x,y,z) is x proportional to |Žł(x,y,z)|2 dxdydz
|Žł(x,y,z)|2 is known as probability density and is always positive.
Related Topics