Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Energies of orbitals
Energies of orbitals
In hydrogen atom, only one electron is present. For such one electron system, the energy of the electron in the nth orbit is given by the expression
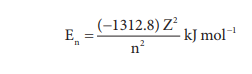
From this equation, we know that the energy depends only on the value of principal quantum number. As the n value increases the energy of the orbital also increases. The energies of various orbitals will be in the following order:
1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d = 4f < 5s = 5p = 5d = 5f < 6s = 6p = 6d = 6f < 7s
The electron in the hydrogen atom occupies the 1s orbital that has the lowest energy. This state is called ground state. When this electron gains some energy, it moves to the higher energy orbitals such as 2s, 2p etc… These states are called excited states.
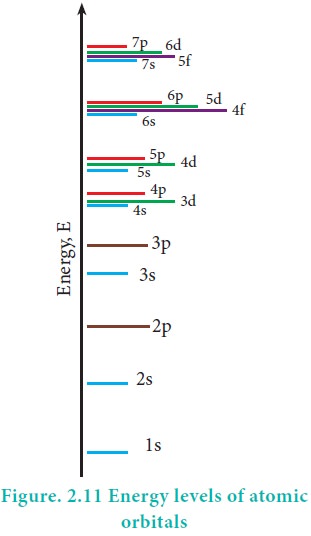
However, the above order is not true for atoms other than hydrogen (multi-electron systems). For such systems the Schrödinger equation is quite complex. For these systems the relative order of energies of various orbitals is given approximately by the (n+l) rule. It states that, the lower the value of (n + l) for an orbital, the lower is its energy. If two orbitals have the same value of (n + l), the orbital with lower value of n will have the lower energy. Using this rule the order of energies of various orbitals can be expressed as follows.
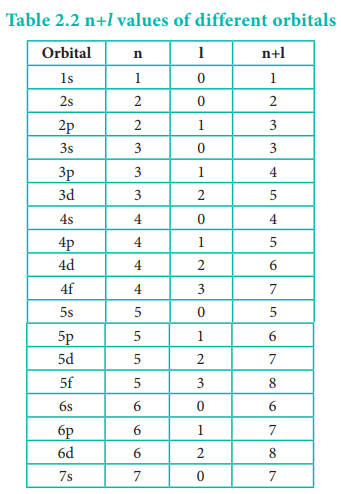
Based on the (n+l) rule, the increasing order of energies of orbitals is as follows:
1s < 2s < 2p < 3s < 3p < 4s <3d <4p <5s<4d < 5p < 6s<4f< 5d < 6p < 7s < 5f < 6d
As we know there are three different orientations in space that are possible for a p orbital. All the three p orbitals, namely, px, py and pz have same energies and are called degenerate orbitals. However, in the presence of magnetic or electric field the degeneracy is lost.
In a multi-electron atom, in addition to the electrostatic attractive force between the electron and nucleus, there exists a repulsive force among the electrons. These two forces are operating in the opposite direction. This results in the decrease in the nuclear force of attraction on electron. The net charge experienced by the electron is called effective nuclear charge. The effective nuclear charge depends on the shape of the orbitals and it decreases with increase in azimuthal quantum number l. The order of the effective nuclear charge felt by a electron in an orbital within the given shell is s > p > d > f. Greater the effective nuclear charge, greater is the stability of the orbital. Hence, within a given energy level, the energy of the orbitals are in the following order. s < p < d < f.
The energies of same orbital decrease with an increase in the atomic number. For example, the energy of the 2s orbital of hydrogen atom is greater than that of 2s orbital of lithium and that of lithium is greater than that of sodium and so on, that is, E2s(H) > E2s(Li) > E2s(Na) > E2s(K).
Related Topics