Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
HeisenbergŌĆÖs uncertainty principle
HeisenbergŌĆÖs
uncertainty principle
The dual nature of matter imposes a limitation on the
simultaneous determination of position and velocity of a microscopic particle.
Based on this, Heisenberg arrived at his uncertainty principle, which states
that ŌĆśIt is impossible to accurately
determine both the position as well as the momentum of a microscopic particle simultaneouslyŌĆÖ. The product of
uncertainty (error) in the measurement is expressed as follows.
╬öx.╬öp Ōēź h/4ŽĆ -------- (2.11)
where Δx and Δp are uncertainties in determining the
position and momentum, respectively.
The uncertainty principle has negligible effect for
macroscopic objects and becomes significant only for microscopic particles such
as electrons. Let us understand this by calculating the uncertainty in the
velocity of the electron in hydrogen atom. (Bohr radius of 1st orbit
is 0.529 Ū║) Assuming that the position of the electron in this orbit is
determined with the accuracy of 0.5 % of the radius
Uncertainity in position = Ōłåx
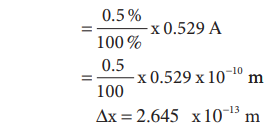
From the HeisenbergŌĆÖs uncertainity principle,
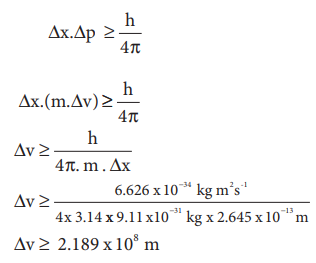
Therefore, the uncertainty in the velocity of the electron
is comparable with the velocity of light. At this high level of uncertainty it
is very difficult to find out the exact velocity.
Related Topics