Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Quantisation of angular momentum and de Broglie concept
Quantisation of angular momentum and de Broglie concept:
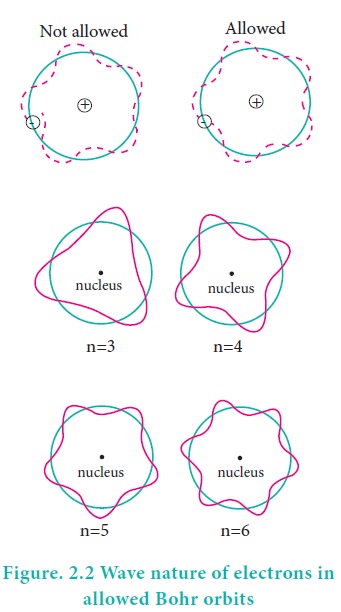
According to the de Broglie concept, the electron that revolves around the nucleus exhibits both particle and wave character. In order for the electron wave to exist in phase, the circumference of the orbit should be an integral multiple of the wavelength of the electron wave. Otherwise, the electron wave is out of phase.
Circumference of the orbit = nλ
2πr = nλ ------------(2.10)
2Ď€r = nh/mv
Rearranging, mvr = nh/2Ď€ ----------(2.1)
Angular momentum = nh/2Ď€
The above equation was already predicted by Bohr. Hence, De Broglie and Bohr’s concepts are in agreement with each other.
Davison and Germer experiment :
The wave nature of electron was experimentally confirmed by Davisson and Germer. They allowed the accelerated beam of electrons to fall on a nickel crystal and recorded the diffraction pattern. The resultant diffraction pattern is similar to the x-ray diffraction pattern. The finding of wave nature of electron leads to the development of various experimental techniques such as electron microscope, low energy electron diffraction etc…
Related Topics