Chapter: Essential Microbiology: Microbial Metabolism
Principles of energy generation
Principles of
energy generation
In this section, we shall consider how
enzyme-catalysed reactions are involved in the cellular capture and utilisation
of energy.
Energy taken up by the cell, be it in the form of
nutrients or sunlight, must be converted into a usable form. A simple analogy
is selling goods for cash, which you can then use to buy exactly what you want.
The ‘cash’ of cellular metabolism is a compound called adenosine triphosphate (ATP). ATP is by far the most important of a
class of compounds known as high-energy transfer compounds, which store the
energy* from the breakdown of nutrients
(or trapped by photosynthetic pigments) and release it when required by the
cell. In catabolic reactions, in
which molecules such as glucose are broken down, energy is released in the form
of ATP, which can then be utilised in anabolic
(synthetic) reactions.
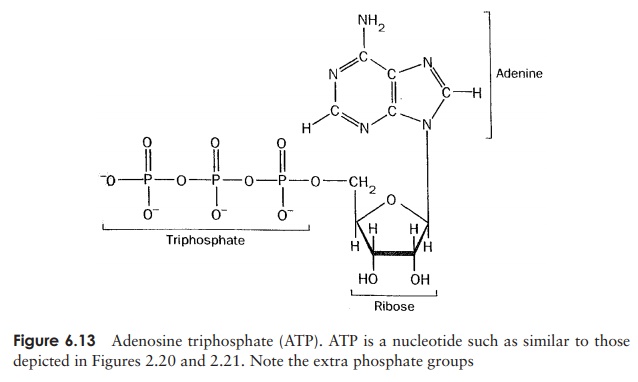
ATP has a structure very similar to the nucleotides
found in RNA, except it has two additional phosphate groups (Figure 6.13). The
bond that links the third phos-phate group requires a lot of energy for its
formation, and is often referred to as a ‘high-energy’
phosphate bond. Importantly for the cell, when this bond is broken, thesame
large amount of energy is released, so when ATP is broken down to ADP and a
free phosphate group, energy is made available to the cell. It should be noted
that ‘high-energy’ refers to the amount of energy needed to make or break the
bond, and not to any intrinsic property of the bond itself. The process of
adding or removing a phosphate group is called phosphorylation or dephosphorylation,
respectively.
Related Topics