Chapter: Essential Microbiology: Microbial Metabolism
Oxidative phosphorylation and the electron transport chain
Oxidative
phosphorylation and the electron transport chain
The components of the electron transport chain differ
between procaryotes and eu-caryotes, and even among bacterial systems, thus
details may differ from the example outlined below. The purpose of the electron
transport is the same for all systems, how-ever, that is, the transfer of
electrons from NADH/FADH2 via a series of carriers to,
ultimately, oxygen. Around half of the energy released during this process is
conserved as ATP.
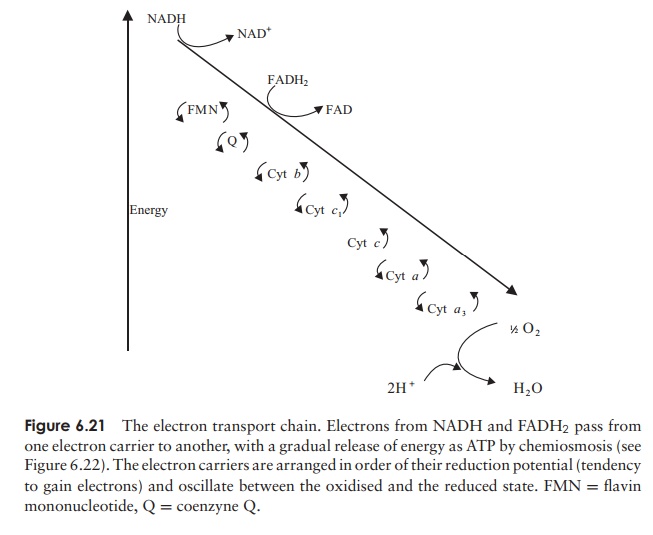
The carrier molecules, which act alternately as
acceptors and donors of electrons, are mostly complex modified proteins such as
flavoproteins and cytochromes, together with a class of lipid-soluble molecules
called ubiquinones (also called coenzyme Q). The carriers are arranged in the
chain such that each one has a more positive redox potential than the previous
one. In the first step in the chain, NADH passes electrons to flavin
mononucleotide (FMN), and in so doing becomes converted back to NAD+ , thereby
ensuring a ready supply of the latter for the continuation of glycolysis
(Figure 6.21). From FMN, the electrons are transferred to coenzyme Q, and
thence to a series of cytochromes; at each transfer of electrons the donor
reverts back to its oxidised form, ready to pick up more electrons. You may
recall that FADH2 yields only two, rather than
three molecules of ATP per molecule; this is because it enters the electron
transport chain at a later point than NADH, thereby missing one of the points
where export of protons occurs. The final cytochrome in the chain transfers its
electrons to molecular oxygen, which, as we have seen, acts as the terminal
oxygen acceptor. The negatively charged oxygen combines with protons from its
surroundings to form water. Four electrons and protons are required for the
formation of each water molecule:
O2+ 4e−+ 4H+−→ 2H2O
Since two electrons are released by the oxidation of
each NADH, it follows that two NADH are needed for the oxidation of each
oxygen.
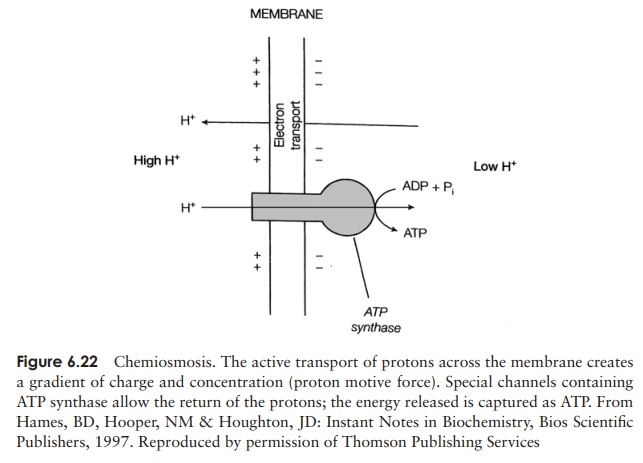
How does this transfer of electrons lead to the
formation of ATP? The chemiosmotictheory proposed
by Peter Mitchell in 1961 offers an explanation. Although it was notimmediately
accepted, the validity of the chemiosmotic model is now widely recognised, and
in 1978 Mitchell received a Nobel Prize for his work. As envisaged by Mitchell,
sufficient energy is released at three points in the electron transport chain
for the transfer of protons to the outside of the membrane, resulting in a
gradient of both concentration and charge (proton
motive force). The protons are able to return across the membrane and
achieve an equilibrium through specific protein channels within the enzyme ATP
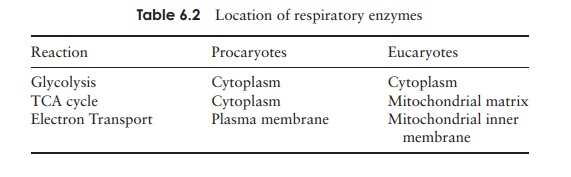
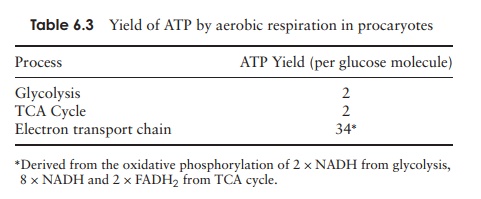
Aerobic respiration in eucaryotes is slightly less
efficient than in procaryotes due to the fact that the three stages take place
at separate locations (see Table 6.2). Thus the total number of ATPs generated
is 36 rather than the 38 in procaryotes (Table 6.3).
Related Topics