Chapter: Essential Microbiology: Microbial Metabolism
Anabolic reactions
Anabolic reactions
So far, in describing respiration and photosynthesis, we have considered those reactions whereby a microorganism may generate cellular energy from its environment. As we saw, one of the uses to which this may be put is the synthesis of new cellular materials. In all of the pathways described in the following section, the conversion of ATP to ADP is required at some point. The term biosynthesis is used to describe those reactions by which nutrients are incorporated first into small molecules such as amino acids and sugars and subsequently into biomacromolecules such as proteins and polysaccharides.
Biosynthesis of carbohydrates
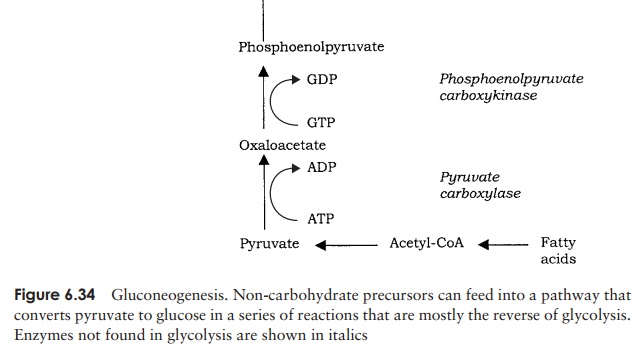
We have already seen in the preceding pages that autotrophic organisms (not necessarily phototrophic ones) are able to incorporate inorganic carbon as CO2 or HCO3− into hexose sugars, most commonly via the Calvin cycle. Heterotrophic organisms are unable to do this, and must convert a range of organic compounds into glucose by a series of reactions called gluconeogenesis (Figure 6.34). Many compounds such as lactate or certain amino acids can be converted to pyruvate directly or via other members of the TCA cycle, and thence to glucose. To all intents and purposes, gluconeogenesis reverses the steps of glycolysis , although not all the enzymes involved are exactly the same. This is because three of the reactions are essentially irreversible, so different enzymes must be used to overcome this. These reactions are highlighted in Figure 6.34.
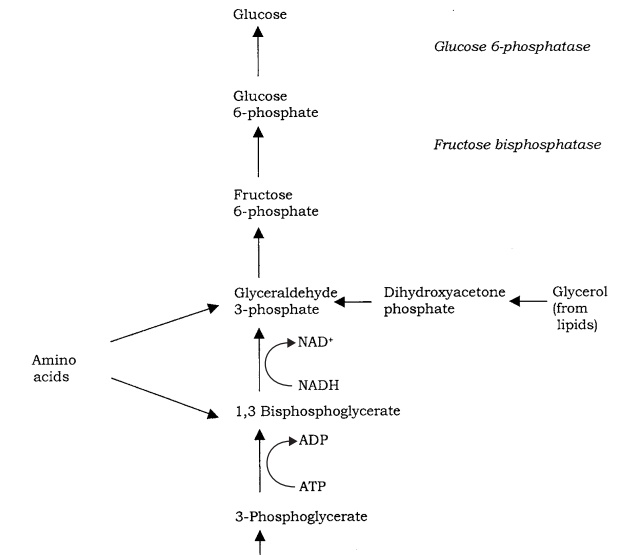
Once glucose or fructose has been produced, it can be converted to other hexose sugars by simple rearrangement reactions. Building up these sugars into bigger carbo-hydrates (polysaccharides) requires them to be in an energised form: this usually takes the form of either an ADP- or UDP-sugar, and necessitates an input of energy. Pentose sugars such as ribose are important in the synthesis of nucleotides for nucleic acids and coenzymes .
Biosynthesis of lipids
Fatty acids are synthesised by a stepwise process that involves the addition of two-carbon units to form a chain, most commonly of 16–18 carbons. The starting point of fatty acid metabolism is the two-carbon compound
The basic building blocks in the synthesis of fatty acids are acetyl-CoA (two-carbon) and malonyl-CoA (three-carbon). We have encountered acetyl-CoA before, when dis-cussing the TCA cycle; malonyl-CoA is formed by the carboxylation of acetyl-CoA.
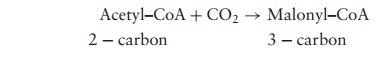
Acetyl–CoA + CO2 → Malonyl–CoA
2 − carbon 3 − carbon
Carbon dioxide is essential for this step, but is not incorporated into the fatty acid as it is removed in a subsequent decarboxylation step. In order to take part in the biosynthesis of fatty acids, both molecules have their coenzyme A element replaced by an acyl carrierprotein (ACP). In a condensation reaction, one carbon is lost as CO2and one of theACPs is released. The resulting four-carbon molecule is reduced, with the involvement of two NADPH molecules, to butyryl-ACP. This is then extended two carbon atoms at a time by a series of further condensations with malonyl-ACP (Figure 6.35).
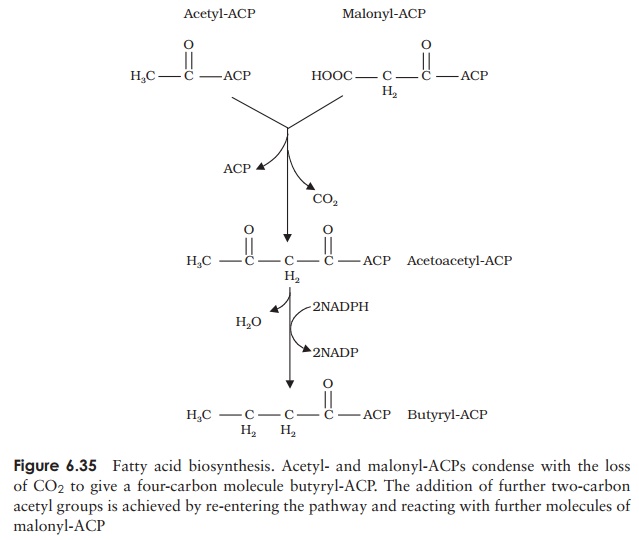
Thus, extending a fatty acid chain by two carbons involves the expenditure of one ATP and two NADPH molecules. The overall equation for the synthesis of a 16-carbon
8 Acetyl-CoA + 7 ATP + 14 NADPH + 6H+−→ Palmitate + 14 NADP++ 8 CoA + 6 H2O + 7 ADP + 7 Pi
Once formed, fatty acids may be incorporated into phospholipids, the major form of lipid found in microbial cells. Recall that a phospholipid molecule has three parts: fatty acid, glycerol and phosphate. These last two are provided in the form of glycerol phosphate, which derives from the dihydroxyacetone phosphate of glycol-ysis. Glycerol phosphate replaces the ACP of two fatty acid-ACP conjugates to yield phosphatidic acid, an important precursor for a variety of membrane lipids. The energy for this reaction is provided, unusually, not by ATP but by CTP (cytidine triphosphate).
Biosynthesis of nucleic acids
Most microorganisms are able to synthesise the purines and pyrimidines that comprise the nitrogenous bases of DNA and RNA. These compounds are synthesised from a number of sources in reactions that require an input of ATP. The contribution of different compounds towards the purine skeleton of guanine or adenine is shown in Figure 6.36.
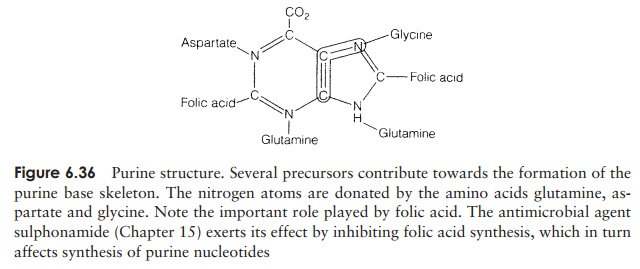
The purines are not synthesised as free bases but are associated with ribose-5-phosphate as complete nucleotides from the outset. Inosinic acid, which is formed initially, acts as a precursor for the other purine nucleotides.
Pyrimidines have a similarly complex synthesis. The amino acids aspartate and glu-tamine are involved in the synthesis of the precursor orotic acid. Note that unlike the purines, the skeleton of pyrimidines is fully formed before association with the ribose-5-phosphate moiety, which is itself derived from glucose (see biosynthesis of carbohy-drates, above).
Ribonucleotides (as contained in RNA) are converted to deoxyribonucleotides (as contained in DNA) by a reduction reaction that may involve vitamin B12 acting as a cofactor.
Biosynthesis of amino acids
A very limited number of microorganisms are able to utilise molecular nitrogen from the atmosphere by incorporating it initially into ammonia and subsequently into or-ganic compounds. Most organisms, however, need to have their ni-trogen supplied as nitrate, nitrite, or ammonia itself. Ammonia can be incorporated into organic nitrogen compounds in several ways, including glutamate formation from α-ketoglutarate (see TCA cycle, above):
α-ketoglutarate+NH4++NADPH*+H+−→glutamate+NADP++H2O
(*Some species utilise NADH as their electron donor)
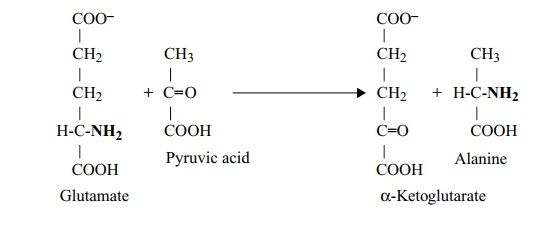
The amino group can subsequently be transferred from the glutamate to make other amino acids by transamination reactions involving other keto acids:
e.g. glutamate + oxaloacetate −→ aspartate +α-ketoglutarate
Glutamate plays a central role in the biosynthesis of other amino acids, as it usually donates the primary amino group of each:
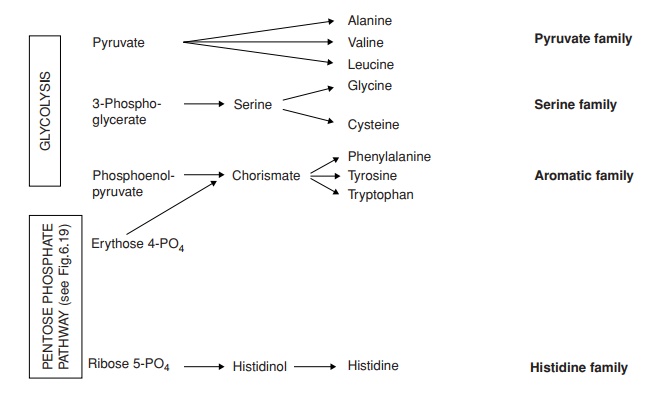
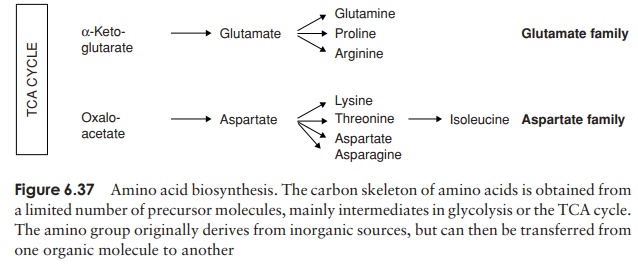
According to the precursor molecule from which they derive, amino acids can be placed into six ‘families’ (Figure 6.37). When
Related Topics