Chapter: Essential Microbiology: Microbial Metabolism
Energy may be generated by the oxidation of inorganic molecules
Energy may be
generated by the oxidation of inorganic molecules
In the previous pages, we have seen how electrons derived from a
variety of organic sources can be channelled into the glycolytic pathway (or
one of its alternatives), and how energy is generated by either oxygen or an
organic/inorganic molecule acting as an electron acceptor. Certain bacteria,
however, are able to derive their energy from the oxidation of inorganic
substrates; these are termed chemolithotrophs.
Molecules such as NH4+ , NO2− , Fe2+ and S0 can be oxidised, with the concomitant
generation of ATP.
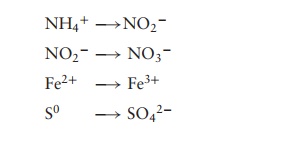
The DG (change in free energy) for each
of these reactions is much smaller than that for aerobic respiration. The value
of DG is a measure of how much energy
is released by a reaction. Thus bacteria using this form of metabolismneed to
oxidise a larger amount of their substrate in order to synthesise the same
amount of cellular material. In most cases, such bacteria are autotrophs,
fix-ing carbon from carbon dioxide via the Calvin
cycle. This is also used by phototrophic organisms, and is de-scribed at
greater length in the section on photosynthe-sis below. If organic carbon is
available, however, someorganisms are able to act as heterotrophs, deriving
their carbon, but not energy, from such molecules. The overall energy yield
from inorganic oxidation is much lower than that from aerobic respiration.
Related Topics