Chapter: Basic & Clinical Pharmacology : General Anesthetics
Pharmacokinetics - Inhaled Anesthetics
PHARMACOKINETICS
Inhaled
anesthetics, volatile as well as gaseous, are taken up through gas exchange in
the alveoli. Uptake from the alveoli into the blood and distribution and
partitioning into the effect com-partments are important determinants of the
kinetics of these agents. As previously mentioned, an ideal anesthetic should
have a rapid onset (induction), and its effect should be rapidly termi-nated.
To achieve this, the effect site concentration in the CNS (brain and spinal
cord) will need to change rapidly. Several factors determine how quickly the
CNS concentration changes.
Uptake & Distribution
A. Inspired Concentration and Ventilation
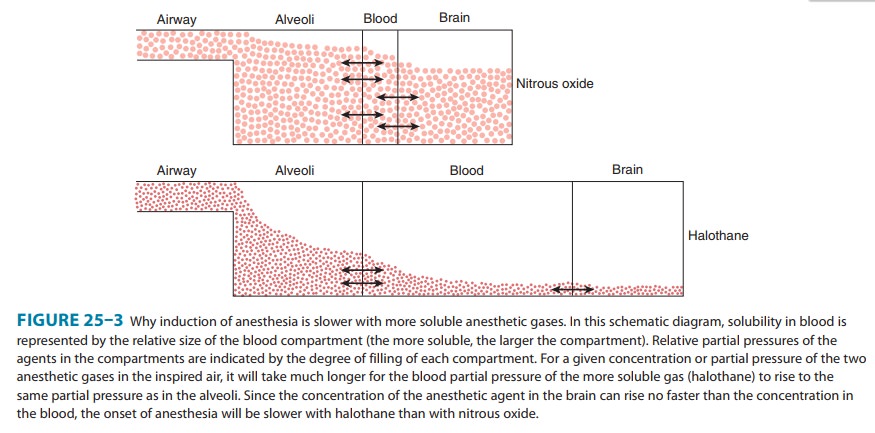
The driving force for uptake of an inhaled anesthetic is the alveo-lar concentration. Two parameters that can be controlled by the anesthesiologist determine how quickly the alveolar concentration changes: (1) inspired concentration or partial pressure, and (2) alveolarventilation. The partial pressure of an inhaled anesthetic in theinspired gas mixture directly affects the maximum partial pressure that can be achieved in the alveoli and the rate of increase of the partial pressure in the alveoli and, ultimately, the blood.
Increases in the
inspired partial pressure increase the rate of rise in the alveoli and thus
accelerate induction. The increase of partial pressure in the alveoli is
usually expressed as a ratio of alveolar concentration (FA) over
inspired concentration (FI); the faster FA/FIapproaches
1 (1 representing the equilibrium), the faster anesthe-sia will occur during an
inhaled induction.The other parameter that directly controls the rate by which
FA/FI approaches 1 is alveolar ventilation. An increase
in ventilation will increase the rate of rise. The magnitude of the effect
varies
according
to the blood:gas partition coefficient. An increase in pulmonary ventilation is
accompanied by only a slight increase in arterial tension of an anesthetic with
low blood solubility, but can significantly increase tension of agents with
moderate to high blood solubility (Figure 25–3). For example, a fourfold
increase in the ventilation rate almost doubles the FA/FI
ratio for halothane during the first 10 minutes of administration but increases
the FA/FI ratio for nitrous oxide by only 15%. Thus,
hyperventilation increases the speed of induction of anesthesia with inhaled
anes-thetics that would normally have a slow onset. Depression of res-piration
by opioid analgesics slows the onset of anesthesia of inhaled anesthetics
unless ventilation is manually or mechanically assisted.
B. Factors Controlling Uptake
The
increase of FA/FI, which is an important determinant of
the speed of induction, is opposed by the
uptake of anesthetic into the
blood, which is
determined by pharmacokinetic parameters unique to the anesthetic
agent as well as patient factors.
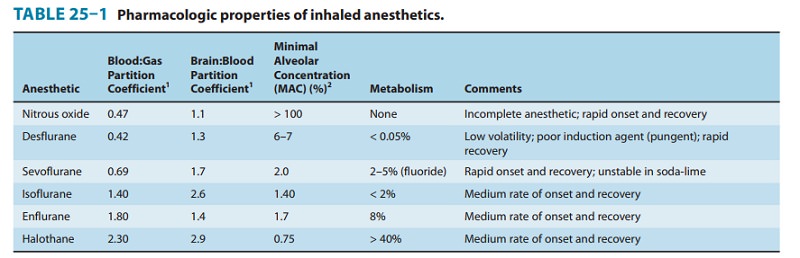
Solubility—One of the most important factors influencingthe transfer of an anesthetic from the lungs to the arterial blood is its solubility characteristics (Table 25–1). The blood:gas partition coefficient is a useful index of solubility and defines the relative affinity of an anesthetic for the blood compared with that of inspired gas. The partition coefficients for desflurane and nitrous oxide, which are relatively insoluble in blood, are extremely low. When an anesthetic with low blood solubility diffuses from the lung into the arterial blood, relatively few molecules are required to raise its partial pressure; therefore, the arterial tension rises rapidly (Figure 25–4, top; nitrous oxide, desflurane, sevoflurane). Conversely, for anesthetics with moderate to high solubility (Figure 25–4, bottom; halothane, isoflurane), more molecules dissolve before partial pressure changes significantly, and arterial tension of the gas increases less rapidly.
A blood:gas partition coef-ficient of 0.47 for nitrous
oxide means that at equilibrium, the concentration in blood is 0.47 times the
concentration in the alveolar space (gas). A larger blood:gas partition coefficient
pro-duces a greater uptake of anesthetic and therefore reduces the time
required for FA/FI to approach 1 (equilibrium, Figure
25–4).
Cardiac output— Changes in pulmonary blood flow haveobvious effects on the uptake of anesthetic gases from the alveolar space. An increase in pulmonary blood flow (ie, increased cardiacoutput) will increase the uptake of anesthetic, thereby decreasing the rate by which FA/FI rises, which will decrease the rate of induc-tion of anesthesia. To better understand this mechanism, one should think about the effect of cardiac output in combination with the tissue distribution and uptake of anesthetic into other tissue compartments.
An increase in cardiac output and pulmonary blood flow will
increase uptake of anesthetic into the blood, but the anesthetic taken up will
be distributed in all tissues, not just the CNS. Cerebral blood flow is well
regulated and the increased cardiac output will therefore increase delivery of
anesthetic to other tissues and not the brain.
Alveolar-venous partial pressure
difference—The
anes-thetic partial pressure difference between alveolar and mixed venous blood
is dependent mainly on uptake of the anesthetic by the tissues, including
nonneural tissues. Depending on the rate and extent of tissue uptake, venous
blood returning to the lungs may contain significantly less anesthetic than
arterial blood. The greater this difference in anesthetic gas tensions, the
more time it will take to achieve equilibrium with brain tissue. Anesthetic
uptake into tissues is influenced by factors similar to those that determine
transfer of the anesthetic from the lung to the intravas-cular space, including
tissue:blood partition coefficients, rates of blood flow to the tissues, and
concentration gradients.
During the induction phase of anesthesia (and the initial phase of the maintenance period), the tissues that exert greatest influ-ence on the arteriovenous anesthetic concentration gradient are those that are highly perfused (eg, brain, heart, liver, kidneys, and splanchnic bed). Combined, these tissues receive over 75% of the resting cardiac output. In the case of volatile anesthetics with rela-tively high solubility in highly perfused tissues, venous blood concentration initially is very low, and equilibrium with the alveo-lar space is achieved slowly.During maintenance of anesthesia with inhaled anesthetics, the drug continues to be transferred between various tissues at rates dependent on the solubility of the agent, the concentration gradi-ent between the blood and the tissue, and the tissue blood flow. Although muscle and skin constitute 50% of the total body mass, anesthetics accumulate more slowly in these tissues than in highly perfused tissues (eg, brain) because they receive only one fifth of the resting cardiac output. Although most anesthetic agents are highly soluble in adipose (fatty) tissues, the relatively low blood perfusion to these tissues delays accumulation, and equilibrium is unlikely to occur with most anesthetics during a typical 1- to 3-hour operation.
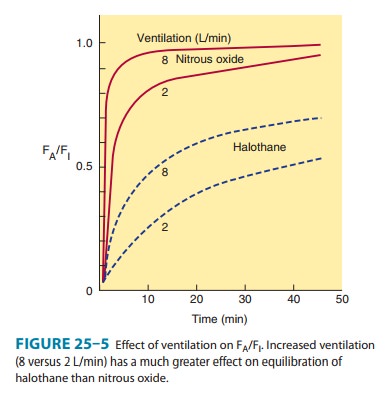
The
combined effect of ventilation, solubility in the different tissues, cardiac
output, and blood flow distribution determines the rate of rise of FA/FI
characteristic of each drug (Figure 25–5). When inducing anesthesia by
inhalation only, the rate by which FA/FI approaches 1
will determine the speed of induction.
Elimination
Recovery
from inhalation anesthesia follows some of the same principles in reverse that
are important during induction. The time to recovery from inhalation anesthesia
depends on the rate of elimination of the anesthetic from the brain. One of the
most important factors governing rate of recovery is the blood:gas parti-tion coefficient
of the anesthetic agent. Other factors controlling rate of recovery include
pulmonary blood flow, magnitude of ventilation, and tissue solubility of the
anesthetic. Two features differentiate the recovery phase from the induction
phase. First, transfer of an anesthetic from the lungs to blood can be enhanced
by increasing its concentration in inspired air, but the reverse transfer
process cannot be enhanced because the concentration in the lungs cannot be
reduced below zero. Second, at the beginning of the recovery phase, the
anesthetic gas tension in different tissues may be quite variable, depending on
the specific agent and the duration of anesthesia. In contrast, at the start of
induction of anesthesia the initial anesthetic tension is zero in all tissues.
Inhaled
anesthetics that are relatively insoluble in blood (ie, possess low blood:gas
partition coefficients) and brain are elimi-nated at faster rates than the more
soluble anesthetics. The washoutof nitrous oxide, desflurane, and sevoflurane
occurs at a rapid rate, leading to a more rapid recovery from their anesthetic
effects com-pared with halothane and isoflurane. Halothane is approximately
twice as soluble in brain tissue and five times more soluble in blood than
nitrous oxide and desflurane; its elimination therefore takes place more
slowly, and recovery from halothane- and isoflu-rane-based anesthesia is
predictably less rapid.
The
duration of exposure to the anesthetic can also have a sig-nificant effect on
the recovery time, especially in the case of the more soluble anesthetics (eg,
halothane and isoflurane). Accumulation of anesthetics in muscle, skin, and fat
increases with prolonged exposure (especially in obese patients), and blood
tension may decline slowly during recovery as the anesthetic is slowly
elimi-nated from these tissues. Although recovery may be rapid even with the
more soluble agents following a short period of exposure, recovery is slow
after prolonged administration of halothane or isoflurane.
A. Ventilation
Two
parameters that can be manipulated by the anesthesiologist are useful in
controlling the speed of induction of and recovery from inhaled anesthesia: (1)
concentration of anesthetic in the inspired gas and (2) alveolar ventilation.
Because the concentra-tion of anesthetic in the inspired gas cannot be reduced
below zero, ventilation is the only way to speed recovery.
B. Metabolism
Modern
inhaled anesthetics are eliminated mainly by ventilation and are only
metabolized to a very small extent; thus, metabolism of these drugs does not
play a significant role in the termination of their effect. However, metabolism
may have important implications for their toxicity (see Toxicity of Anesthetic
Agents). Hepatic metabolism may also contribute to the elimination of and recovery
from some older volatile anesthetics. For example, halothane is eliminated more
rapidly during recovery than enflurane, which would not be predicted from their
respective tissue solubility. This increased elimination occurs because over
40% of inspired halo-thane is metabolized during an average anesthetic
procedure, whereas less than 10% of enflurane is metabolized over the same
period.
In
terms of the extent of hepatic metabolism, the rank order for the inhaled
anesthetics is halothane > enflurane > sevoflurane > isoflurane >
desflurane > nitrous oxide (Table 25–1). Nitrous oxide is not metabolized by
human tissues. However, bacteria in the gastrointestinal tract may be able to
break down the nitrous oxide molecule.
Related Topics