Chapter: Basic & Clinical Pharmacology : General Anesthetics
Ketamine - Intravenous Anesthetics
KETAMINE
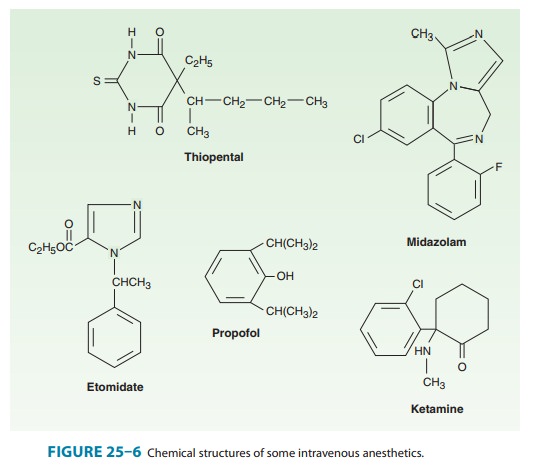
Ketamine
(Figure 25–6) is a partially water-soluble and highly lipid-soluble
phencyclidine derivative differing from most other intrave-nous anesthetics in
that it produces significant analgesia. The characteristic state observed after
an induction dose of ketamine is known as “dissociative anesthesia,” wherein
the patient’s eyes remain open with a slow nystagmic gaze (cataleptic state).
Of the two ste-reoisomers the S (+)
form is more potent than the R (–)
isomer, but only the racemic mixture of ketamine is available in the USA.
Ketamine’s
mechanism of action is complex, but the major effect is probably produced
through inhibition of the NMDA receptor complex.
Pharmacokinetics
The
high lipid solubility of ketamine ensures a rapid onset of its effect. As with
other intravenous induction drugs, the effect of a single bolus injection is
terminated by redistribution to inactivetissue sites. Metabolism occurs
primarily in the liver and involves N-demethylation
by the cytochrome P450 system. Norketamine,the primary active metabolite, is
less potent (one third to one fifth the potency of ketamine) and is
subsequently hydroxylated and conjugated into water-soluble inactive
metabolites that are excreted in urine. Ketamine is the only intravenous
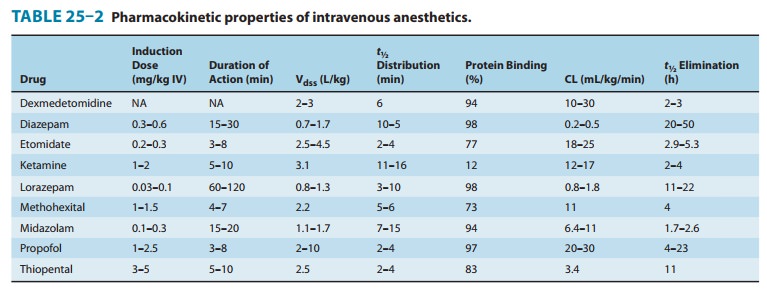
anesthetic that has low protein binding (12%) (Table 25–2).
Organ System Effects
If
ketamine is administered as the sole anesthetic, amnesia is not as complete as
with the benzodiazepines. Reflexes are often preserved, but it cannot be
assumed that patients are able to protect the upper airway. The eyes remain
open and the pupils are moderately dilated with a nystagmic gaze. Frequently,
lacrimation and salivation are increased, and pre-medication with an
anticholinergic drug may be indicated to limit this effect.
A. CNS Effects
In
contrast to other intravenous anesthetics, ketamine is considered to be a
cerebral vasodilator that increases
cerebral blood flow, as well as CMRO2. For these reasons, ketamine
has traditionally not been recommended for use in patients with intracranial
pathology, especially increased ICP. Nevertheless, these perceived undesirable
effects on cerebral blood flow may be blunted by the maintenance of
normocapnia. Despite the potential to produce myoclonic activity, ketamine is
considered an anticonvulsant and may be recommended for treatment of status
epilepticus when more conventional drugs are ineffective.Unpleasant emergence
reactions after administration are the main factor limiting ketamine’s use.
Such reactions may include vivid colorful dreams, hallucinations, out-of-body
experiences, and increased and distorted visual, tactile, and auditory
sensitivity. These reactions can be associated with fear and confusion, but a
euphoric state may also be induced, which explains the potential for abuse of
the drug. Children usually have a lower incidence of and less severe emergence
reactions. Combination with a benzodi-azepine may be indicated to limit the
unpleasant emergence reac-tions and also increase amnesia.
B. Cardiovascular Effects
Ketamine
can produce transient but significant increases
in sys-temic blood pressure, heart rate, and cardiac output, presumably by
centrally mediated sympathetic stimulation. These effects, which are associated
with increased cardiac workload and myocar-dial oxygen consumption, are not
always desirable and can be blunted by coadministration of benzodiazepines,
opioids, or inhaled anesthetics. Though the effect is more controversial,
ket-amine is considered to be a direct myocardial depressant. This property is
usually masked by its stimulation of the sympathetic nervous system but may
become apparent in critically ill patients with limited ability to increase
their sympathetic nervous system activity.
C. Respiratory Effects
Ketamine
is not thought to produce significant respiratory depres-sion. When it is used
as a single drug, the respiratory response to hypercapnia is preserved and
blood gases remain stable. Transient hypoventilation and, in rare cases, a
short period of apnea can follow rapid administration of a large intravenous
dose for induc-tion of anesthesia. The ability to protect the upper airway in
the presence of ketamine cannot be assumed despite the presence of active
airway reflexes. Especially in children, the risk for laryngo-spasm because of
increased salivation must be considered; this risk can be reduced by
premedication with an anticholinergic drug. Ketamine relaxes bronchial smooth
muscles and may be helpful in patients with reactive airways and in the
management of patients experiencing bronchoconstriction.
Clinical Uses & Dosage
Its
unique properties, including profound analgesia, stimulation of the sympathetic
nervous system, bronchodilation, and minimal respiratory depression, make
ketamine an important alternative to the other intravenous anesthetics and a
desirable adjunct in many cases despite the unpleasent psychotomimetic effects.
Moreover, ketamine can be administered by multiple routes (intravenous,
intramuscular, oral, rectal, epidural), thus making it a useful option for premedication
in mentally challenged and uncoopera-tive pediatric patients.Induction of
anesthesia can be achieved with ketamine, 1–2 mg/kg intravenously or 4–6 mg/kg
intramuscularly. Though the drug is not commonly used for maintenance of
anesthesia, its short con-text-sensitive half-time makes ketamine a candidate
for this pur-pose. For example, general anesthesia can be achieved with the
infusion of ketamine, 15–45 mcg/kg/min, plus 50–70% nitrous oxide or by
ketamine alone, 30–90 mcg/kg/min.Small bolus doses of ketamine (0.2–0.8 mg/kg
IV) may be useful during regional anesthesia when additional analgesia is
needed (eg, cesarean delivery under neuraxial anesthesia with an insufficient
regional block). Ketamine provides effective analgesia without compromise of
the airway. An infusion of a subanalgesic dose of ketamine (3–5 mcg/kg/min)
during general anesthesia and in the early postoperative period may be useful
to produce analge-sia or reduce opioid tolerance and opioid-induced
hyperalgesia. The use of ketamine has always been limited by its unpleasant
psychotomimetic side effects, but its unique features make it a very valuable
alternative in certain settings, mostly because of the potent analgesia with
minimal respiratory depression. Most recently it has become popular as an
adjunct administered at subanalgesic doses to limit or reverse opioid
tolerance.
Related Topics