Chapter: Clinical Anesthesiology: Anesthetic Management: Pediatric Anesthesia
Pediatric Anesthesia: Pharmacological Differences
PHARMACOLOGICAL DIFFERENCES
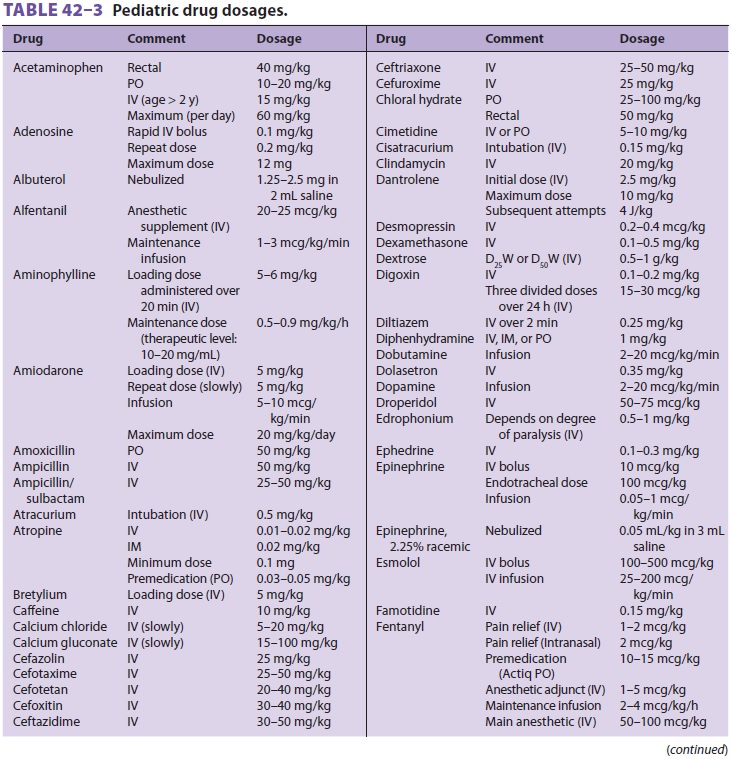
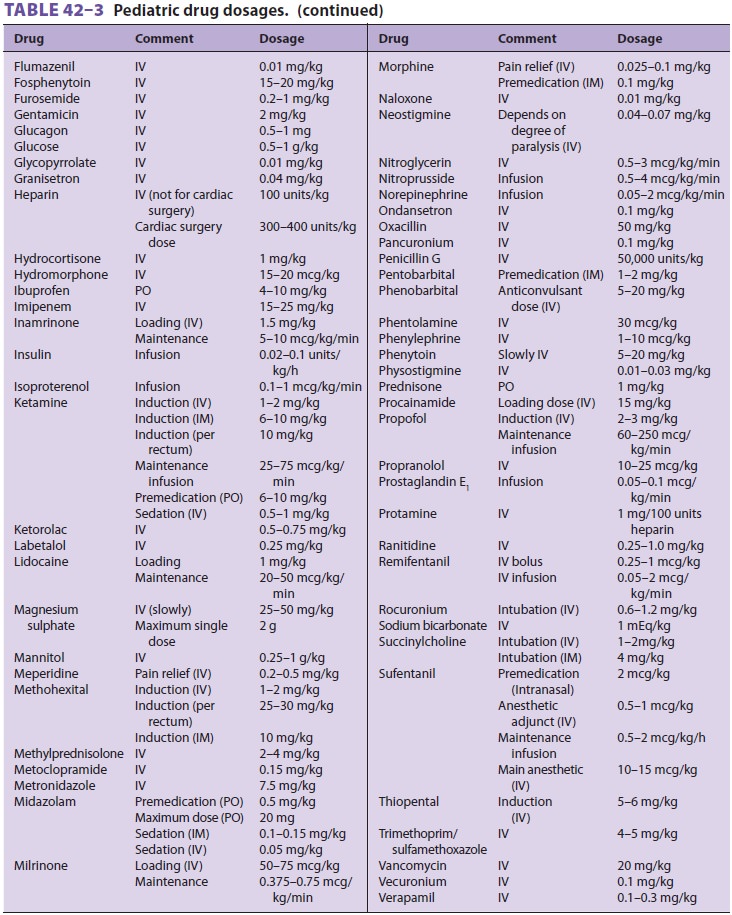
Pediatric drug dosing is typically adjusted on a per-kilogram basis for
convenience (Table 42–3). In
early childhood a patient’s weight can be approxi-mated based on age:
50th percentile weight (kg) = (Age × 2) + 9
Weight-adjustment of drug dosing is
incom-pletely effective because it does not take into account the
disproportionately larger pediatric intravas-cular and extracellular fluid
compartments, the immaturity of hepatic biotransformation pathways, increased
organ blood flow, decreased protein for drug binding, or higher metabolic rate.
Neonates and infants have a proportionately
greater total water content (70–75%) than adults (50–60%). Total body water
content decreases while fat and muscle content increase with age. As a direct
result, the volume of distribution for most intra-venous drugs is
disproportionately greater in neo-nates, infants, and young children, and the
optimal dose (per kilogram) is usually greater than in older children and
adults. A disproportionately smaller muscle mass in neonates prolongs the
clinical dura-tion of action (by delaying redistribution to muscle) of drugs
such as thiopental and fentanyl. Neonates also have a relatively decreased
glomerular filtration rate, hepatic blood flow, and renal tubular function, and
immature hepatic enzyme systems. Increased intraabdominal pressure and
abdominal surgery further reduce hepatic blood flow. All these factors may
impair renal drug handling, hepatic metabo-lism, or biliary excretion of drugs
in neonates and young infants. Neonates also have decreased protein binding for
some drugs, most notably thiopental, bupivacaine, and many antibiotics. In the
case of thiopental, increased free drug enhances potency and reduces the
induction dose in neonates com-pared with older children. An increase in free
bupi-vacaine might increase the risk of systemic toxicity.
Inhalational Anesthetics
Neonates, infants, and young children have
relatively greater alveolar ventilation andreduced FRC compared with older
children and adults. This greater minute ventilation-to-FRC ratio with
relatively greater blood flow to vessel-rich organs contributes to a rapid
increase in alveolar anesthetic concentration and speeds inhalation induction.
Furthermore, the blood/gas coefficients of volatile anesthetics are reduced in
neonates com-pared with adults, resulting in even faster induction times and
potentially increasing the risk of acciden-tal overdosage.
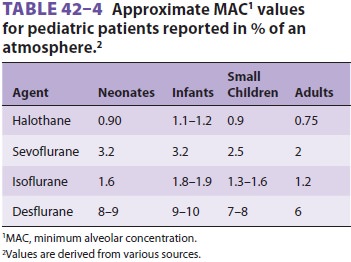
The minimum alveolar concentration (MAC) for
halogenated agents is greater in infantsthan in neonates and adults (Table 42–4). In con-trast to other agents, no increase in sevoflurane MAC can be
demonstrated in neonates and infants. Nitrous oxide does not appear to reduce
the MAC of desflurane or sevoflurane in children to the same extent as it does
for other agents.
The blood pressure of neonates and infants
appears to be especially sensitive to volatile anesthetics. This clinical
observation has been attributed to less-well-developed compensatory mechanisms
(eg, vasoconstriction, tachycardia) and greater sensitivity of the immature
myocar-dium to myocardial depressants. Halothane (now much less commonly used)
sensitizes the heart to catecholamines. The maximum recommended dose of
epinephrine in local anesthetic solutions during halothane anesthesia is 10
mcg/kg. Cardiovascular depression, bradycardia, and arrhythmias are less
frequent with sevoflurane than with halothane. Halothane and sevoflurane are less
likely than other volatile agents to irritate the airway or cause breath
holding or laryngospasm during induction . In general, volatile anesthetics
appear to depress ventilation more in infants than in older children.
Sevoflurane appears to produce the least respiratory depression. The risk for
hal-othane-induced hepatic dysfunction appears to be much reduced in
prepubertal children compared with adults. There are no reported instances of
renal toxicity attributed to inorganic fluoride pro-duction during sevoflurane
anesthesia in children.Overall, sevoflurane appears to have a greater
ther-apeutic index than halothane and has become the preferred agent for
inhaled induction in pediatric anesthesia.
Emergence is fastest following desflurane or sevoflurane, but both
agents are associated with a greater incidence of agitation or delirium upon
emergence, particularly in young children. Because of the latter, some
clinicians switch to isoflurane for maintenance anesthesia following a
sevoflurane induction .
Nonvolatile Anesthetics
After weight-adjustment of dosing, infants
and young children require larger doses of propofol because of a larger volume
of distribution compared with adults. Children also have a shorter elimina-tion
half-life and higher plasma clearance for propofol. Recovery from a single
bolus is not appre-ciably different from that in adults; however, recov-ery
following a continuous infusion may be more rapid. For the same reasons,
children may require increased weight-adjusted rates of infusion for
maintenance of anesthesia (up to 250 mcg/kg/min). Propofol is not recommended
for prolonged seda-tion of critically ill pediatric patients in the intensive
care unit (ICU) due to an association with greater mortality than other agents.
Although the “propofol infusion syndrome” has been reported more often in
critically ill children, it has also been reported in adults undergoing
long-term propofol infusion (>48 h) for sedation, particularly at increased doses (>5 mg/kg/h). Its
essential features include rhabdo-myolysis, metabolic acidosis, hemodynamic
insta-bility, hepatomegaly, and multiorgan failure.
Children require relatively larger doses of
thio-pental compared with adults. The elimination half-life is shorter and the
plasma clearance is greater than in adults. In contrast, neonates, appear to be
more sensitive to barbiturates. Neonates have less pro-tein binding, a longer
half-life, and impaired clear-ance. The thiopental induction dose for neonates
is 3–4 mg/kg compared with 5–6 mg/kg for infants.
Opioids appear to be more potent in neo-nates than in older children and
adults. Unproven (but popular) explanations include “easier entry” across the
blood–brain barrier, decreased metabolic
capability, or increased sensitivity of the
respiratory centers. Morphine sulfate, particularly in repeated doses, should
be used with caution in neonates because hepatic conjugation is reduced and
renal clearance of morphine metabolites is decreased. The cytochrome P-450
pathways mature at the end of the neonatal period. Older pediatric patients
have relatively greater rates of biotransformation and elimination as a result
of high hepatic blood flow. Sufentanil, alfentanil, and, possibly, fentanyl
clear-ances may be greater in children than in adults. Remifentanil clearance
is increased in neonates and infants but elimination half-life is unaltered
com-pared with adults. Neonates and infants may be more resistant to the hypnotic
effects of ketamine, requiring slightly higher doses than adults (but the
“differences” are within the range of error in studies); pharmacokinetic values
do not appear to be signifi-cantly different from those of adults. Etomidate
has not been well-studied in pediatric patients younger than 10 years of age;
its profile in older children is similar to that in adults. Midazolam has the
fastest clearance of all the benzodiazepines; however, mid-azolam clearance is
significantly reduced in neonates compared with older children. The combination
of midazolam and fentanyl can cause hypotension in patients of all ages.
Muscle Relaxants
For a wide variety of reasons (including
pharmacol-ogy, convenience, case mix, and convenience), muscle relaxants are
less commonly used during induction of anesthesia in pediatric than in adult
patients. Many children will have a laryngeal mask airway (LMA) or endotracheal
tube placed after receiving a sevoflurane inhalation induction, placement of an
intravenous catheter, and administration of various combinations of propofol,
opioids, or lidocaine.
All muscle relaxants generally have a faster
onset (up to 50% less delay) in pediatric patients because of shorter
circulation times than adults. In both children and adults, intravenous
succinyl-choline (1–1.5 mg/kg) has the fastest onset . Infants require
significantly larger doses of succinylcholine (2–3 mg/kg) than older children
and adults because of the relatively larger volume of distribution. This
discrepancy disappears
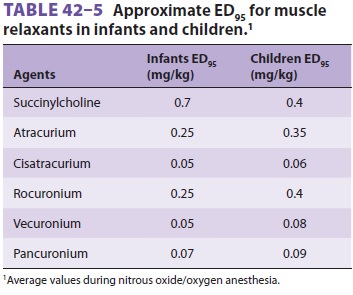
if dosage is based on body surface area. Table 42–5 lists commonly used muscle relaxants and their ED95 (the dose that produces 95% depression of
evoked twitches). With the notable exclusion of succinyl-choline and possibly
cisatracurium, infants require significantly smaller muscle relaxant doses than
older children. Moreover, based on weight, older children require larger doses
than adults for some neuromuscular blocking agents (eg, atracurium). As with
adults, a more rapid intubation can be achieved with a muscle relaxant dose
that is twice the ED 95 dose at the expense of prolonging the duration of action.
The response of neonates to nondepolarizing
muscle relaxants is variable. Popular (and unproven) explanations for this
include “immaturity of the neuromuscular junction” (in premature neonates),
tending to increase sensitivity (unproven), counter-balanced by a
disproportionately larger extracellular compartment, reducing drug
concentrations (proven). The relative immaturity of neonatal hepatic function
prolongs the duration of action for drugs that depend primarily on hepatic
metabolism (eg, pancuronium, vecuronium, and rocuronium). Atracurium and
cisatracurium do not depend on hepatic
biotransformation and reliably behave as intermediate-acting muscle relaxants.
Children are more susceptible than adults to
cardiac arrhythmias, hyperkalemia, rhabdomyolysis, myoglobinemia, masseter
spasm, and malignant hyperthermia
associated with succinylcholine. When a child experiences cardiac arrest
following administration of succinylcho-line, immediate treatment for
hyperkalemia should be instituted. Prolonged, heroic (eg, potentially including
cardiopulmonary bypass) resuscitative efforts may be required. For this reason,
succinylcho-line is avoided for routine, elective paralysis for intubation in children and adolescents.
Unlike adults, children may have profound bradycardia and sinus node arrest
following the first dose of succinylcholine without atropine pretreatment.
Atropine (0.1 mg minimum) must therefore always be administered prior to
succinylcholine in children. Generally accepted indications for intravenous
suc-cinylcholine in children include rapid sequence induction with a “full”
stomach and laryngospasm that does not respond to positive-pressure
ventila-tion. When rapid muscle relaxation is required prior to intravenous
access (eg, with inhaled inductions in patients with full stomachs),
intramuscular succinyl-choline (4–6 mg/kg) can be used. Intramuscular atropine (0.02
mg/kg) should be administered with intramuscular succinylcholine to reduce the
likeli-hood of bradycardia. Some clinicians advocate intra-lingual
administration (2 mg/kg in the midline to avoid hematoma formation) as an
alternate emer-gency route for intramuscular succinylcholine.
Many clinicians consider rocuronium (0.6 mg/ kg intravenously) to be the
drug of choice (when a relaxant will be used) during routine intubation in
pediatric patients with intravenous access because it has the fastest onset of
nondepolarizing neuro-muscular blocking agents . Larger doses of rocuronium
(0.9–1.2 mg/kg) may be used for rapid sequence induction but a prolonged
dura-tion (up to 90 min) will likely follow. Rocuronium is the only
nondepolarizing neuromuscular blocker that has been adequately studied for
intramuscular administration (1.0–1.5 mg/kg), but this approach requires 3–4
min for onset.
Atracurium or cisatracurium may be preferred in young infants,
particularly for short procedures, because these drugs consistently display
short to intermediate duration.
As with adults, the effect of incremental doses of muscle relaxants
(usually 25–30% of the initial dose) should be monitored with a peripheral nerve stimulator. Sensitivity can vary significantly
between patients. Nondepolarizing blockade can be reversed with neostigmine
(0.03–0.07 mg/kg) or edropho-nium (0.5–1 mg/kg) along with an anticholinergic
agent (glycopyrrolate, 0.01 mg/kg, or atropine, 0.01– 0.02 mg/kg). Sugammadex,
a specific antagonist for rocuronium and vecuronium, has yet to be released in
the United States.
Related Topics