Chapter: Modern Pharmacology with Clinical Applications: Pharmacological Management of Chronic Heart Failure
Myocardial ExcitationŌĆōContraction Coupling
MYOCARDIAL EXCITATIONŌĆōCONTRACTION
COUPLING
The physiological processes
that begin with cardiac sar-colemmal membrane depolarization and culminate in
contraction are collectively defined as myocardial
exci-tationŌĆōcontraction coupling. Depolarization of the car-diac myocyte
sarcolemmal membrane during the action potential results in the intracellular
entry of extracellu-lar calcium. The major regulators of the transsarcolem-mal
entry of calcium include L-type calcium channels and autonomic receptors (Fig.
15.1). These membrane-bound proteins all contribute to the influx of a minute
quantity of calcium from outside the cell into the myo-cyte. The entry of this
small quantity of calcium causes the release of the large reservoir of calcium
stored in the sarcoplasmic reticulum (SR) through the SR cal-cium release
channel (ryanodine receptor). This large reservoir of calcium interacts with
tropomyosin to allow the actin and myosin filaments to overlap, resulting in
systolic myocardial contraction. Diastolic relaxation re-sults from the
resequestration of this large reservoir of calcium back into the sarcoplasmic
reticulum through the SR calcium adenosine triphosphatase (ATPase). Calcium
exits the cell through the NA+ ŌĆōCa++ exchanger and
sarcolemmal Ca++ ATPase.
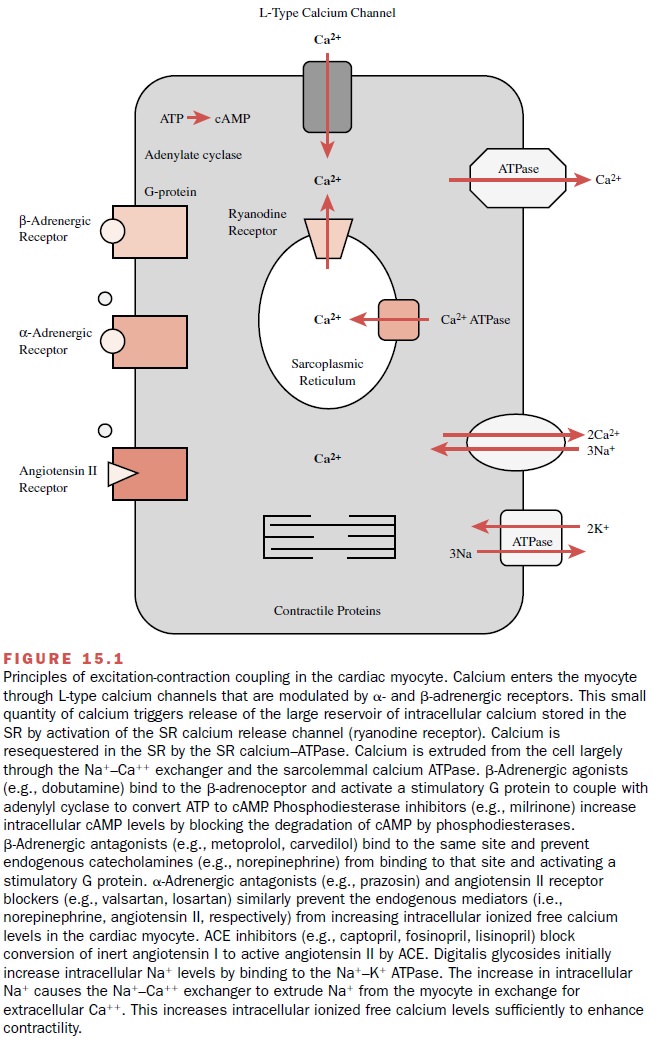
Autonomic receptors further
regulate calcium influx through the sarcolemma (Fig. 15.1). ╬▓-Adrenergic stim-ulation
results in the association of a catalytic subunit of a G protein coupled to the
╬▓-receptor. This stimulates
the enzyme adenylyl cyclase to convert ATP to cyclic adenosine monophosphate
(cAMP). Increasing cAMP production results in a cAMP-dependent phosphoryla-tion
of the L-type calcium channel and a subsequent in-crease in the probability of
the open state of the chan-nel. This translates to an increase in
transsarcolemmal calcium influx during phase 2 (the plateau phase) of the
cardiac muscle action potential. The effects of transient increases in
intracellular levels of cAMP are tightly con-trolled by phosphodiesterases and
phosphatases that prevent indefinite phosphorylation and activation of
regulatory proteins. ╬▒-Adrenoceptor stimulation results in the phospholipase CŌĆōmediated
breakdown of phos-phatidylcholine to inositol triphosphate and diacyl
glyc-erol; these second messengers further enhance mobi-lization of both
transsarcolemmal calcium influx and SR calcium efflux.
Binding of angiotensin II to
its cardiac myocyte re-ceptor acutely increases Ca++ influx through
sarcolem-mal L-type calcium channels. The long-term effects of chronic angiotensin
II receptor stimulation include car-diac myocyte hypertrophy through enhanced
expres-sion of growth factor genes.
The maintenance of a resting
membrane potential in cardiac myocytes, as well as all cells, depends on
meta-bolic energy (ATP) that is used by the NA+ ŌĆōK+ ATPase
to drive the gradients for NA+ and K+ between the
in-tracellular and extracellular spaces. Cardiac glycosides are known to bind
to this protein.
Related Topics