Chapter: Genetics and Molecular Biology: Transposable Genetic Elements
Mu Phage As a Giant Transposable Element
Mu Phage As a Giant Transposable Element
A transposon could carry DNA with phage-like
functions rather than a simple antibiotic resistance gene. Such a transposon
would be able to travel freely from cell to cell autonomously as a virus. Phage
Mu first drew attention by its ability to generate mutations in infected cell
populations. These mutations were generated by its insertion into various
bacterial genes. Careful mapping of many Mu phage insertions into the β-galactosidase gene showed that Mu inserted without
high specificity in its target sequence. Thus, Mu behaved like a lambda phage
with little sequence specificity in its choice of the bacterial att site.
Two facts, however, indicate that Mu is more like a
transposon than like phage lambda. First, Mu duplicates five bases of
chromosomal DNA upon its insertion. Most transposons generate such
duplications. These arise as a result of staggered nicking of the target
sequence followed by insertion of the transposon and replication (Fig. 19.9).
Second, Mu does not excise from the chromosome and replicate in the cytoplasm.
Even though an induced cell may yield a hundred Mu phage upon lysis, never
during the lytic cycle are any free Mu DNA molecules observed. The Mu DNA is
replicated only by transposition. Packaging of the Mu takes place directly on
the integrated DNA. A dramatic demonstration of replication by transposition is
provided by Southern transfers of DNA taken from a Mu lysogen before and after
induction of the phage. Before induction, only a single restriction fragment of
the host DNA contains sequences
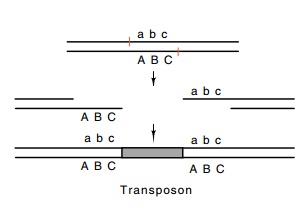
Figure
19.9 Insertion of atransposon into a
site with staggered nicks followed by filling in of the gaps generates a
duplication of the se-quence included between the nicks.
homologous to a restriction fragment including the
end of Mu, but half an hour after induction of Mu growth, many restriction
fragments contain these sequences (Fig. 19.10).
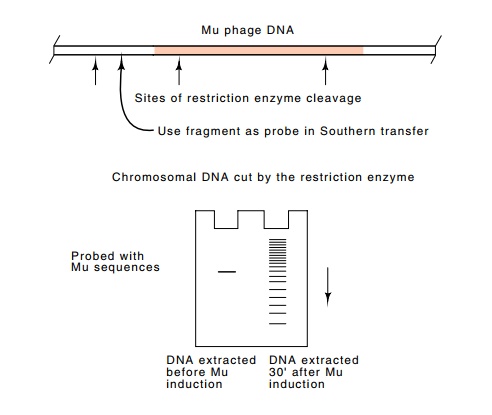
Mu packages its DNA right out of the chromosomal
insertions. Part of the head structure recognizes a sequence near the left end
of the DNA, reaches out beyond the phage 50 to 150 base pairs, and begins
packaging. A headfull of DNA is packaged, and the remaining DNA is then
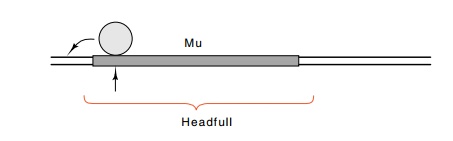
cleaved and the tail is attached. Since a Mu phage
headfull of DNA is slightly larger than the genome of the phage, the packaged
DNA usually extends beyond the right end of the phage and includes about 3,000
base pairs of bacterial DNA as well. The headfull hypothesis explains the fact that
a Mu phage carrying an insertion packages a smaller amount of bacterial DNA
roughly equal in amount to the size of the insertion.
The sequence heterogeneity that results from the
headfull mode of packaging is dramatically displayed when heteroduplexes of
phage genomes are analyzed in the electron microscope. Since it is highly
unlikely that the two strands from the same phage will rehybridize upon
formation of the heteroduplex, two unrelated strands will associate. The phage
DNA portions of the strands will, of course, be complementary and will form a
duplex. The strands of bacterial DNA on the right end, however, are unlikely to
be complementary, and these will remain single-stranded and are observed as
“split ends.” The similar split ends from the left end are too small to be
clearly observed.
Related Topics