Chapter: Genetics and Molecular Biology: Transposable Genetic Elements
Hopping by Tn10
Hopping by Tn10
An intermediate in the transposition process of Tn3
contains both the source and target molecules joined together in a cointegrate.
Then a recombination event in the res
site resolves these structures. The exist-ence of cointegrates proves that Tn3
transposes by replication. Transposon Tn10 is different. No cointegrate
structures were ever observed. This fact suggests that Tn10 might transpose
without repli-cation.
A conceptually simple experiment can test whether
Tn10 replicates as it transposes. Kleckner and Bender constructed a lambda
phage that could not replicate upon entering a normal host. The phage contained
Tn10 that carried both a tetracycline resistance gene and the lacZ gene. A second phage was also made
that was identical to the first except that it contained a nonsense mutation in
lacZ.
Consider the consequences of infecting a LacZ- cell
with a phage that is heteroduplex for the point mutation (Fig. 19.15). Since
the phage does not replicate, the only way for cells to become tetracycline
resistant is for the transposon to enter the cell’s chromosome. If the
transposon
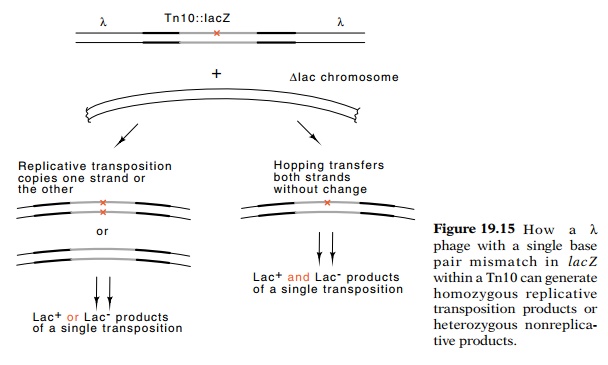
replicates as it transposes, then the lacZ that is inserted into the
chro-mosome will be homozygous. It will either be wild type or mutant on both
strands. If, however, the transposon excises itself from the phage and inserts
itself into the chromosome before replicating, then the lacZ gene will remain heterozygous. If the cells are repair
deficient, only after the first chromosome replication will each DNA molecule
within the cells become homozygous. This means, however, that the descendants
of the original cell will be of two types, Lac+ and Lac-. Because
cells forming a colony do not mix, one half the colony will be Lac+ and one
half Lac-. On Lac indicating plates a sector comprising about half the colony
will indicate Lac+ and the other half will indicate Lac-.
A simple control experiment was possible by
infecting cells lysogenic for lambda phage. The infecting lambda phage could
recombine into the prophage in regions of homology at considerably higher
frequency that the transposon relocates on its own. Therefore,
tetracycline-resis-tant cells would have obtained a heterozygous copy of the lacZ gene, and should show sectoring on
the indicating plates. They did. Therefore Tn10 hops.
In order for Tn10 to survive, it must hop
infrequently, and shortly after passage of a replication fork . The infrequent hopping results
from a low synthesis rate and extreme instability
of its transposase. The transposase is so unstable that the larger the Tn10
element, the less frequently it transposes, and transposase barely func-tions
in trans at all. The low synthesis
rate of transposase results from two factors. One is a low activity of the
promoter serving transposase, pINon IS10-L, and the second is antisense messenger
deriving from pOUT(Fig. 19.16). This second promoter is active, and
its product hybridizes
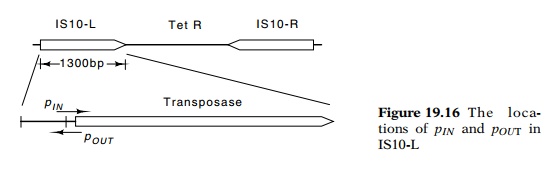
with the beginning of transposase messenger to
block translation. This is one of several examples of antisense messenger
playing an important biological role.
A simple experiment showed that antisense messenger
from pOUT reduces translation of the pIN messenger rather than interfering with its transcription. Two fusions
of β-galactosidase to the transposase gene were made
(Fig. 19.17). One was a protein fusion in which the N-termi-nal portion of
transposase substituted for the N-terminal of β-galactosi-dase. In this, the levels of transposase messenger as well as
any regulation of translation would affect the synthesis of β-galactosidase. In the second fusion, β-galactosidase was placed further downstream so
that only the synthesis level of transposase messenger would affect the
synthesis of β-galactosidase. A plasmid with a
copy of the pOUT region was introduced to these two cell lines. The
extra copies of the pOUT RNA
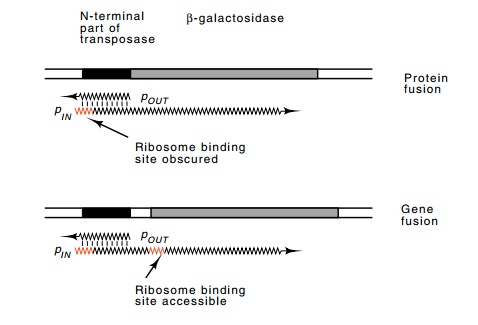
Figure
19.17 Hybridization of transcripts frompOUTtopINinterferes withtranslation of the protein fusion between transposase and
β-galactosidase, but not the gene fusion.
decreased β-galactosidase
synthesis from the protein fusion, but not from the gene fusion, thus showing
that the antisense RNA affects translation of transposase messenger.
Another type of control of transposition also
exists. It has been found that damaging the host cell dam methylase system increases transposi-tion frequency. The dam methylase is the system that
methylates GATC sequences to permit repair enzymes to identify newly replicated
DNA. There are two GATC sequences in IS10, one in the -10 region of pIN, and the other in the binding site of transposase.
Methylating both of these sites greatly decreases the transposition frequency
by reducing both the promoter activity and the transposase activity. What about
half meth-ylated, or hemimethylated DNA, as is generated after replication? Invitro experiments showed that DNA
methylated on one strand is signifi-cantly more active than fully methylated
DNA, both in the promoter activity and in the transposase activity.
The activity of hemimethylated DNA could also be
examined in vivo. Conjugating male
cells transfer only one strand. The recipients synthe-size the complementary
strand as it enters. Donor Dam+ cells were mated with Dam-
recipient cells to generate hemimethylated DNA. This DNA was prevented from
recombining into the female by the presence of a RecA-
mutation. Thus, the only way recipient cells could become tetracycline
resistant would be for the transposon to hop. The frequency of transposition
hopping could be measured by determining the fre-quency of
tetracycline-resistant cells. Hemimethylated Tn10 transposed much more frequently
than fully methylated Tn10. These results mean than Tn10 is much more likely to
transpose just after the replication fork has passed.
Related Topics