Chapter: 11th Microbiology : Chapter 13 : Immunology
Measurement of Antigen and Antibody
Measurement of Antigen and
Antibody
Many methods are available for the measurement of antigens and
antibodies participating in the primary, secondary and tertiary reactions.
Measurement may be in terms of mass (Example: mg Nitrogen) or
morecommonlyasunitsortitre.Theantibody titre of a serum is the highest dilution
of the serum which gives an observable reaction with the antigen in the
particular test. The titre of a serum is influenced by the nature and quantity
of the antigen and the type and conditions of the test. Antigens may also be
titrated against sera.
Two important parameters of serological tests are sensitivity
and specificity. Sensitivity refers to the ability
of the test to detect even very minute quantities of antigen and antibody. When
a test is highly sensitive, false negative results will be absent or minimal. Specificity refers to the ability of the test to detect reactions
between homologus antigens and antibodies only. When a test is highly specific,
false positive results will be absent or minimal. Some tests are qualitative
and others are quantitative. The various tests used for detection of antigen
and antibodies are given below:
1.
Precipitation tests
2.
Agglutintion tests
3.
Complement Fixation test
4.
Immunofluorescence
5.
Radio immuno assay
6.
Enzyme linked immuno sorbent assay
7.
Western Blotting technique
8.
Neutralization test
In this section Agglutination and Precipitation reactions will
be described in detail.
1. Precipitation reactions
When a soluble antigen combines with its antibody in the
presence of electrolytes (NaCl) at a suitable temperature and pH, the
antigen-antibody complex, forms an insoluble (visible) precipitate and this
reaction is called precipitation. When instead of
sedimenting, the precipitate remains suspended as floccules, the reaction is
known as flocculation.
Applications of precipitation reactions
The following types of precipitation tests are in common use:
a) Ring test
This test consists of layering the antigen solution over a
column of antiserum in a narrow tube. A visible precipitate forms at the
junction of the two liquids. Examples of ring precipitation test are the C-
reactive protein test, Ascoli’s thermoprecipitin and the grouping of
streptococci by the Lancefield technique.
b) Slide test
When a drop of antigen and a drop of antiserum are placed on a
slide and mixed by shaking, floccules appear. The VDRL test for syphilis is an
example of slide flocculation.
c) Tube test
A quantitative tube flocculation test is used for the
standardization of toxins and toxoids. Serial dilution of the toxin / toxoid is
added to the tube containing a fixed quantity of the antitoxin. The toxin or
toxoid that flocculates optimally with one unit of the antitoxin is defined as
the Lf (Lethal Flocculation) dose.
Precipitation reaction in gels
There are several advantages in allowing precipitation to occur
in a gel rather than in a liquid medium. The reaction is visible as a distinct
band of precipitation, which is stable and can be stained for preservation, if
necessary. Imunodiffusion is usually performed in 1% agarose gel. Different
modifications of the test are available.
·
Single Diffusion in One Dimension (Oudin Procedure)
·
Double Diffusion in One Dimensions (Oakley-Fulthorpe Procedure)
·
Single Diffusion in Two Dimensions (Mancini Procedure)
·
Double Diffusion in Two Dimensions (Ouchterlony Procedure)
Immunoelectrophoresis
Immunoelectrophoresis was devised by Grabar and Williams (1953).
This method consists of two steps. The first step is agarose electrophoresis of
the antigen. Rectangular trough is then cut into the agarose gel parallel to
the direction of the electric field and is filled with the antiserum. By
diffusion, lines of precipitation develop with each of the separated compounds
(Figure 13.28). This method is used to detect normal and abnormal serum
proteins.
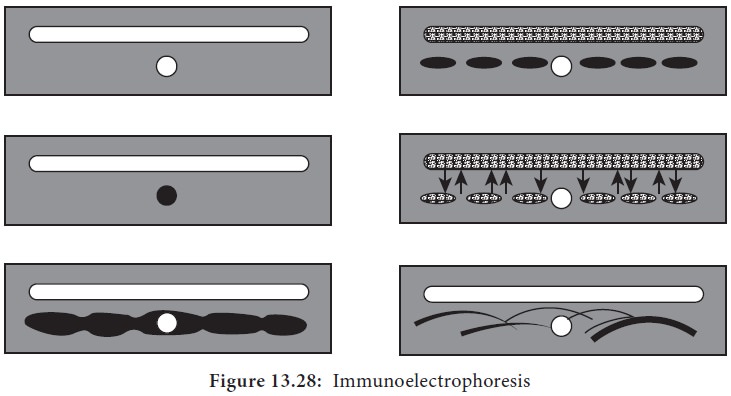
1.
Semisolid agar layered on the glass slide. A well for antigen
and a trough for antiserum cut out of agar.
2.
Antigen well filled with human serum.
3.
Serum separated by electrophoresis.
4.
Antiserum trough filled with antiserum to whole human serum.
5. Serum and antiserum allowed to diffuse into agar.
6.
Precipitin lines form for individual serum proteins
·
Counterimmunoelectrophoresis
·
Rocket Electrophoresis
2. Agglutination reactions
When a particulate antigen is mixed with its antibody in the
presence of electrolytes at a suitable temperature and pH, the particles are
clumped or agglutinated, and the reaction is called agglutination.
Agglutination is more sensitive than precipitation for detection
of antibodies. Agglutination occurs optimally when antigens and antibodies
react in equivalent proportions. Incomplete or monovalent antibodies (having
only one antigen combining site) do not cause agglutination, though they
combine with the antigen. They may act as blocking antibodies inhibiting
agglutination by the complete antibody added subsequently.
Direct agglutination test
In the direct technique, a cell or insoluble
particulate antigen is agglutinated directly by antibody. An example is the
agglutination of group A erythrocytes by anti-A sera.
Indirect (Passive) agglutination test
Passive agglutination refers to
agglu-tination of antigen coated cells or inert particles (bentonite or latex
particles) which are passive carriers of soluble an-tigens. An example is the
latex agglutina-tion for detection of rheumatoid factor. When instead of the
antigen, the antibody is adsorbed to carrier particles in test for estimation
of antigen, this technique is known as reverse passive agglutination.
Hemagglutination inhibition method
The inhibition of agglutination of antigen-coated red blood
cells by homologous antigen is a highly sensitive and specific method for
detecting small quantities of soluble antigen in blood or other tissue fluids.
The principle of this method is that antibody preincubated with soluble
homologous antigen will be inactivated when incubated with antigen coated red
blood cells.
This method is used in the detection of HBs Ag in hepatitis and
in the detection of factor VIII antigen in hemophilia.
Hemagglutination inhibition is also used to detect antibodies
against certain viruses (Arbovirus, Influenza, Measles and Rubella). These
viruses are able to agglutinate red blood cells because they possess
hemagglutinins on their outer surfaces.
Applications of agglutination reactions
a) Slide agglutination
When a drop of the appropriate antiserum is added to a smooth
uniform suspension of a particulate antigen in a drop of saline on a slide,
agglutination takes place. A positive result is indicated by the clumping
together of the particles and the cleaning of the drop. Mixing the antigen and
the antiserum by gently rocking the slide facilitates the reaction.
It is essential to have on the same slide a
control consisting of the antigen suspension in saline, without the antiserum, to
ensure that the antigen is not auto agglutinable. Agglutination is visible to
the naked eye but may sometimes require confirmation under the microscope.
Slide agglutination is a routine test for the identification of many bacterial
isolates from clinical specimens. It is also the method used for blood grouping
and cross matching.
b) Tube agglutination
This is a standard quantitative method for measurement of
antibodies. When a fixed volume of a particulate antigen suspension is added to
an equal volume of serial dilution of an antiserum in test tubes, the agglutination
titre of the serum can be estimated. Widal test done for typhoid and Weil Felix test done for rickettsial infections are examples of Tube agglutination.
Latex agglutination test
Here latex particles are used as passive carriers for adsorbed
soluble antigens. The most widespread application of latex agglutination has
been in the detection of rheumatoid factor. In rheumatoid arthritis, the
patient’s produces rheumatoid factor. Rheumatoid factor is a pentameric IgM
antibody directed against IgG. The test consists of coating latex particles
with IgG and reacting them with the patient serum. Agglutination indicates a
positive test. Latex agglutination tests are also employed in the clinical
laboratory for detection of HBs Ag, ASO (Antistreptolysin O) and CRP
(Carbohydrate Reactive Protein)
Coombs test (antiglobulin test)
This test was devised by Coombs, Mourant and Race (1945) for the
detection of anti-Rh antibodies that do not agglutinate Rh-positive red blood
cells in saline. When sera containing incomplete anti-Rh antibodies are mixed
with Rh- positive red blood cells, the antibody globulin coats the surface of
the red blood cells, though they are not agglutinated. When such red blood
cells coated with antibody globulin are washed free of all unattached protein
and treated with a rabbit antiserum against human gammaglobulin (antiglobulin
or Coombs serum), the cells are agglutinated. This is the principle of the
Coombs test (Figure 13.29).
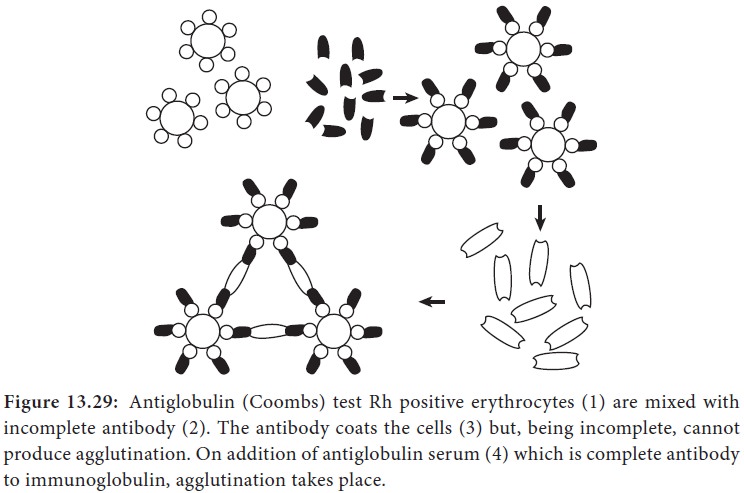
The Coombs test may be of the direct or the indirect type.
Applications of coombs test
1.
Erythrocyte typing in blood banks.
2.
The evaluation of hemolytic disease of the newborn.
3. The diagnosis of autoimmune hemolytic anemia.
Related Topics