Definition, Structure, Function, Properties and Activities - Antibodies | 11th Microbiology : Chapter 13 : Immunology
Chapter: 11th Microbiology : Chapter 13 : Immunology
Antibodies
Antibodies
The first real chemical information regarding the structure of
antibodies was provided by Tiselius and Kabat in the early 1940s. They demonstrated that the gamma globulin
fraction of serum proteins that migrated most slowly in electrophoresis
contained most of the serum antibodies. This section deals with the structural
and biological properties of antibodies (immunoglobulins).
Definition of antibodies
Antibodies are glycoproteins present in serum gamma globulins
produced by B-lymphocytes (B cells) or Plasma cells in response to exposure to antigen. Antibodies are also known as
immunoglobulins. They react specially with that antigen in vivo or in vitro and are hence a part of the adaptive immune response specifically, humoral immunity.
Structure of an Immunoglobulin Molecule
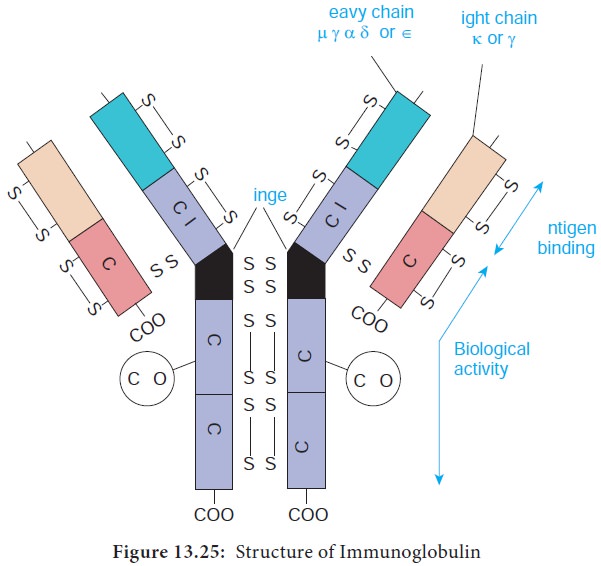
1. Basic unit
The basic structural unit (monomer) of an immunoglobulin
molecule consists of four polypeptide chains linked covalently by disulfide
bonds (Figure 13.25). The four-chain structure is composed of two identical light
(L) and two identical heavy (H) polypeptide chains. Every immunoglobulin can be
represented by the general formula (H2L2)n.
a) Light chains
Light Chains have a molecular weight of approximately 25000 Da and are composed of about 220 amino acids. Light chains are common to all immunoglobulin classes and are of two types – kappa (κ) or lambda(λ) - based on their structural differences. A given immunoglobulin molecule may contain either identical κ or λ chains but never both.
b) Heavy chains
Heavy chains have a molecular weight of approximately twice that
of light chains (57000-70000 Da) and twice the number of amino acids (about
440). Five antigenically distinct isotypes of heavy chains are recognized-gamma
(γ), alpha (α), mu (μ), delta (δ) and epsilon (ε) – based on structural
differences in the carboxy terminal portion of heavy chains. The heavy chains
isotypes form the basis of five classes of immunoglobulin molecules – IgG
(contains γ chain), IgA (contains α chain), IgM (contains μ chain), IgD
(contains δ chain) and IgE (contains ε chain). Five heavy chain classes of
immunoglobulin can be easily remembered as GAMDE. Heavy chain classes are again
subdivided into subclasses of molecules.
1.
Four known subclasses of the γ chain exist – γ1, γ2, γ3 and γ4 -
which yield IgG1, IgG2, IgG3 and IgG4.
2.
Two subclasses of the α chain are known – α1 and α2 - which
yield IgA1 and IgA2.
3.
Two subclasses of the μ chain are known – μ1 and μ2 - which
yield IgM1 and IgM2.
4.
No subclasses of the δ and ε (IgD and IgE) are known.
2. Disulfide bonds
Disulfide bonds hold together the four polypeptide chains in
normal immunoglobulin molecules and are of two types namely interchain bonds and intrachain bonds.
a. Inter chain bonds occur between heavy chains (H-H), heavy and light chains (H-L) and light
chains (L-L). H-H bonds occur primarily in the hinge region and can vary in
number from 1-15 depending on the class and subclass of the immunoglobulin
molecules.
b. Intra chain bonds are stronger than interchain bonds and occur within the individual chain type,
with the number of bonds varying depending on the type (light chains have two,
human γ, α and δ heavy chains have four and human μ and ε heavy chains have
five). The distribution of intrachain disulfide bonds forms the basis for
division of each immunoglobulin into domains.
3. Regions
Each heavy and light chain consists of two segments, the variable region and the constant region. The variable (V) region shows a wide variation in amino acid sequence in the
amino terminal portion of the molecule. The areas of high variability in the
variable region of heavy (VH) and light (VL) chains are called hypervariable regions or complementarity determining
regions (CDRs). Hypervariable regions are most intimately involved in
formation of the antigen binding site.
4. Domains
Each immunoglobulin chain consists of a series of globular
regions enclosed by disulphide bonds. Each heavy chain consists of four or five
domains - one in the variable region (VH ) and three or four in the constant
region (CH1, CH2, CH3, and CH4). Each light chain consists of two domains – one
in the variable region (VL) and one in the constant region (CL).
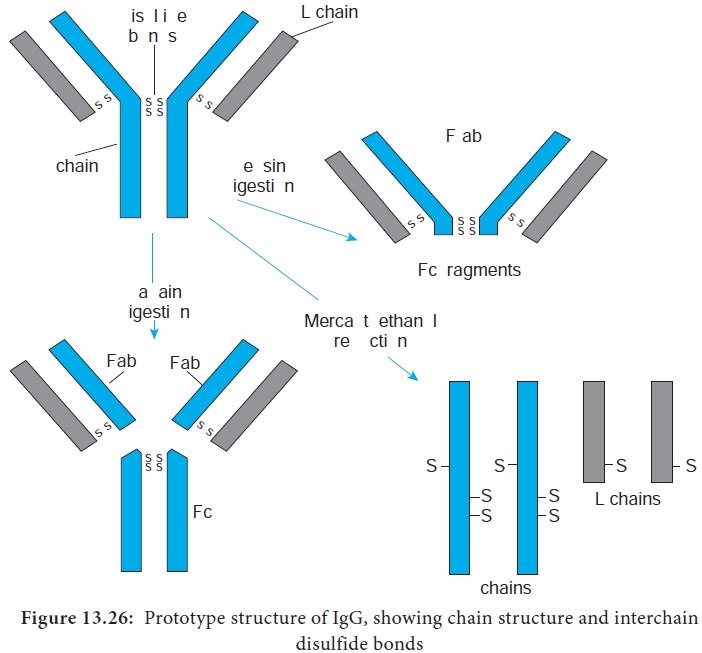
5. Fragments.
Proteolytic (peptide bond -splitting) enzymes such as papain and pepsin are used to degrade
immunoglobulin molecules into definable fragments to facilitate study of their
structure.
i. Treatment of the monomeric basic unit with the enzyme papain
splits it into two Fab fragments (Fragment-antigen binding) and one Fc fragment.
These Fab fragments can bind but cannot precipitate the antigen; therefore,
they are monovalent, possessing only one combining site each.
ii. Treatment of the immunoglobulin molecule with pepsin results
in digestion of most of the Fc fragment, leaving one large fragment that
consists of two Fab fragments joined by covalent bonds, termed the F(ab’)2 fragment. The F(ab’)2 fragments has two antigen
combining sites. Therefore it is bivalent, possessing the ability to bind and
precipitate an antigen (Figure 13.26).
6. Hinge region
Hinge region is the portion of heavy chain between the CH1 and CH2 domains. It is highly flexible and allows for movement of the Fab arms in relation to each other. The S values (sedimentation coefficient that is expressed in Svedberg units(s)) of immunoglobulins range from 7S- 19S.
Immunoglobulin Function
There are three major effector functions that enable antibodies
to remove antigens and kill pathogens. Opsonization promotes antigen
phagocytosis by macrophages and neutrophils. Complement activation by IgM and IgG can
activate a pathway that leads to the generation of a collection of proteins
that can perforate cell membranes. Antibody-dependent cell-mediated
cytotoxicity (ADCC) can cause NK cell mediated death of target cells when
antibody bound to the target cells associates with Fc receptors of natural
killer (NK) cells.
Properties and Activities of Immunoglobulin Classes
Each immunoglobulin class
differs in its general
properties, distribution in the
body and interaction
with other components of the host
defensive systems.
i) IgG
·
IgG is the major immunoglobulin in human serum, accounting for
80% of the immunoglobulin pool.
·
It is present in blood plasma and tissue fluids. It has a
monomeric structure.
·
IgG class acts against bacteria and viruses by opsonizing the
invaders and neutralizing toxins and viruses.
·
IgG molecules are capable of fixing complement, except for IgG4.
·
It is the major antibody in the secondary immune response and it
has half life of 23 days.
·
IgG is the only immunoglobulin molecule able to cross the placenta
and provides natural immunity in utero and to the neonate at birth.
ii) IgA
It is present in the serum and in various bodily secretions and
thus takes two forms – serum IgA and secretory IgA (sIgA)
A) Serum IgA
·
It accounts for about 12% of serum immunoglobulin.
·
In humans, over 80% of serum IgA exists in a monomeric form and
the remaining existing as polymers in the form of dimers, trimers or tetramers.
In polymeric IgA, the monomeric units are linked by disulphide bonds and
joining (J) chain.
·
Serum IgA fixes complement via the alternative pathway. It has a
half life of 5 days.
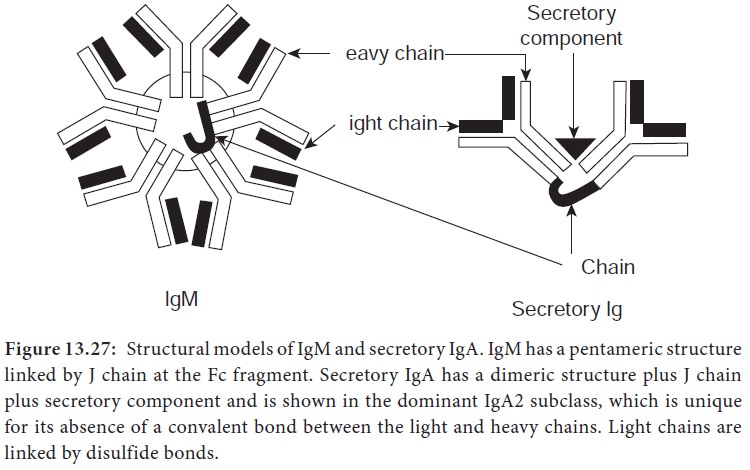
B) Secretory IgA
1.
SecretoryIgA is the primary immunoglobulin of mucosal associated
lymphoid tissue (MALT). It is also found in saliva, tears, and breast milk.
2.
It consists of two monomeric units plus J chain and secretory
component (Figure 13.29).
3.
The dominant subclass of sIgA is sIgA2 which is unique for its
absence of a covalent bond between the light and heavy chains. In this
subclass, light chains are linked by disulphide bonds.
4.
It has a half life of 5-6 days. It is responsible for local
immunity.
5.
The sIgA molecules protect mucosal surfaces by reacting with the
surface of potential pathogens and interfering with their adherence and
colonization. It also plays a role in the alternative complement pathway.
iii) IgM
·
IgM accounts for about 5-10% of the serum immunoglobulin pool.
·
It has a pentameric structure consisting of five monomeric units
linked by J chain and disulphide bonds at the Fc fragment (Figure 13.27).
·
It is the predominant antibody in the primary immune response to
most antigens and the predominant antibody produced by the fetus.
·
It is the first immunoglobulin made during B cell maturation and
individual IgM monomers are expressed on B cells, serving as the antibody component
of the B cell receptor (BCR).
·
IgM tends to remain in the bloodstream, where it agglutinates
(clumps) bacteria, activates complement by the classical pathway and enhances
the ingestion of pathogens by phagocytic cells.
·
It has a half life of approximately 5 days.
iv) IgD
·
IgD accounts for about less than 1% of the total immunoglobulin
pool.
·
One unique structural feature is the presence of only a single
H-H inter chain bond along with two H-L interchain bonds.
·
It has a monomeric structure similar to that of IgG.
·
IgD antibodies are abundant in combination with IgM on the
surface of B cells and thus are part of the B cell receptor complex. Therefore
their function is to signal the B cell to start antibody production upon initial
antigen binding.
·
It has a half life of 2-3 days.
v) IgE
·
IgE accounts for only 0.004% of serum immunoglobulin. It has a
monomeric structure. It is also called reagin or reaginic antibody.
·
Theskin sensitizing and anaphylactic antibodies belong to this
class.
· The Fc portion of IgE can bind to Fc receptors specific for IgE that are found on mast cells, eosinophils and basophils. Thus these cells can become coated with IgE molecules. Whentwocell-boundIgEmolecules are cross linked by binding to the same antigen, the cells degranulate. This degranulation releases histamine and other mediators of inflammation.
·
IgE also stimulates production of an excessive number of
eosinophils in the blood (eosinophilia) and increased rate of movement of the
intestinal contents (gut hypermotility) which aid in the elimination of
helminthic parasites. IgE has a half life of 2-3 days.
Antigenic Determinants on Immunoglobulins
Since antibodies are glycoproteins, they can themselves function
as potent immunogens to induce an antibody response. Such anti-Ig antibodies
are powerful tools for the study of B cell development and humoral immune
response. The antigenic determinants or epitopes, on immunoglobulin molecules
fall into three major categories: isotypic, allotypic and idiotypicdeteminants, which are located in
characteristic portions of the molecule.
Related Topics