Types, Mechanisms, Functions - Immunity | 11th Microbiology : Chapter 13 : Immunology
Chapter: 11th Microbiology : Chapter 13 : Immunology
Immunity
Immunity
To establish an infection, an invading microorganism must first
overcome many surface barriers, such as skin, degradative
These surface barriers have either direct
antimicrobial activity or inhibit attachment of the microorganism to the host.
Any microorganism that penetrates these barriers encounters two levels of
resistance: nonspecific resistance mechanisms and the specific immune response.
Types of Immunity
The term immunity (Latin immunis, free of burden) refers to the general
ability of a host to resist infection or disease. There are two interdependent
components of the immune response to invading microorganisms and foreign
material. They are non-specific immune response or innate immunity or natural immunity and specific immune response or acquired immunity or adaptive immunity.
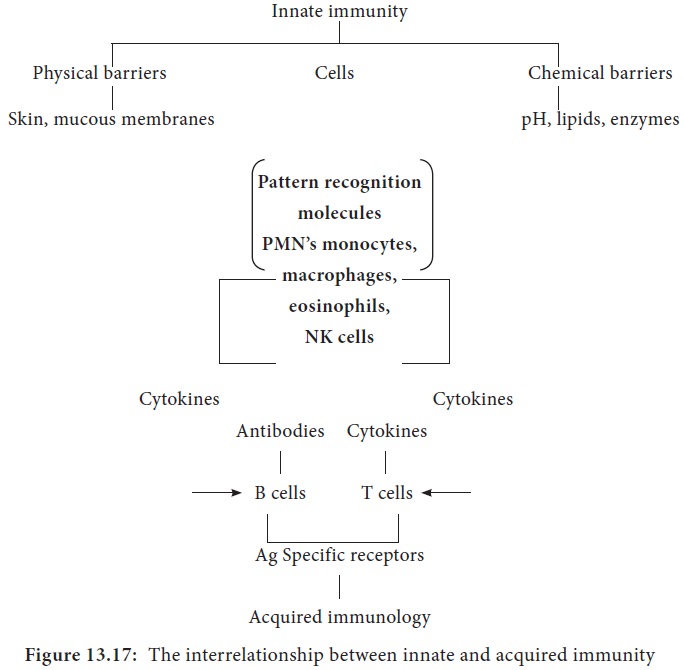
I. Innate immunity
Innate immunity refers to those
general defence mechanisms that are inherited as part of the innate
structure and function of each animal (such as skin, mucus and lysozyme).
Innate immunity is the first line of defence against any microorganism or
foreign material encountered by the vertebrate host. Innate immunity defends
against foreign invaders equally and lacks immunological memory.
II. Acquired immunity
Acquired immunity refers to the type of specific immunity that develops after exposure to a suitable
antigen. The effectiveness of acquired immunity increases on repeated exposure
to foreign agents such as viruses, bacteria or toxins. So acquired immunity has
memory. The innate immunity and acquired immunity work together to eliminate
pathogenic microorganisms and other foreign agents. Although innate systems
predominate immediately upon initial exposure to foreign substances, multiple
bridges occur between innate and acquired immune system components (Figure
13.17).
Mechanisms of Innate Immunity
A potential microbial pathogen invading a human host immediately
confronts a vast array of nonspecific defence mechanisms. Many direct factors
(nutrition, physiology, fever, age, genetics) and equally as many indirect
factors (personal hygiene, socioeconomic status, living conditions) influence
all host microbe relationships. In addition to these direct and indirect
factors, a vertebrate host has the following four non specific defence
mechanisms.
A. Physical barriers
B. Chemical mediators
C. Phagocytosis
D. Inflammation
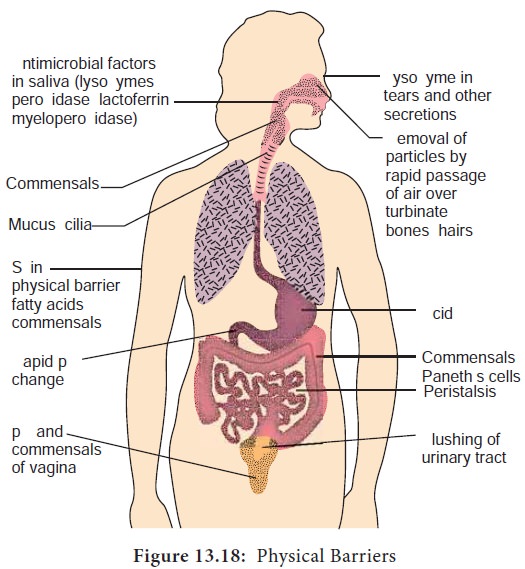
A. Physical barriers
i) Skin
Intact skin contributes greatly to host resistance. It forms a
very effective mechanical barrier to microbial invasion. Its outer layer
consists of thick, closely packed cells called keratinocytes, The skin is slightly
acidic (around pH 5-6) due to skin oil, secretion from sweat glands and organic
acids produced by commensal Staphylococci.
It also contains a high concentration of sodium chloride and is subject to
periodic drying.
ii) Mucous membranes
The mucous membranes of the eye (conjunctiva), the respiratory,
digestive and urogenital systems withstand microbial invasion. The intact
stratified squamous epithelium and mucus secretions form a protective covering
that resists penetration and traps many microorganisms. Many mucosal surfaces
are bathed in specific antimicrobial secretions. One antibacterial substance in
these secretions is lysozyme, an enzyme that lyses
bacteria. Mucous secretions possess the iron binding protein, lactoferrin. Lactoferrin sequesters iron from the plasma reducing the
amount of iron available to invading microbial pathogens and prevents their
ability to multiply. Mucous membranes produce lactoperoxidase, an enzyme that
catalyzes the production of superoxide radicals, reactive oxygen intermediate
that is toxic to many microorganisms.
iii) Respiratory system
The mammalian respiratory system has strong defense mechanisms.
The average person inhales at least eight microorganisms a minute or 10,000
each. Microbes larger than 10μm are trapped by hairs and cilia lining the nasal
cavity. The cilia in the nasal cavity beat toward the pharynx, so that mucus
with its trapped microorganisms is moved toward the mouth and expelled.
Microbes smaller than 10μm pass through the nasal cavity and are trapped by the
mucociliary blanket and the trapped microbes are transported by
ciliary action that moves them away from lungs. Coughing and sneezing reflexes
clear the respiratory system of microorganisms by expelling air forcefully from
the lungs through the mouth and nose, respectively. Salivation also washes
microorganisms from the mouth and nasopharyngeal areas into the stomach.
iv) Gastrointestinal tract
Most microorganisms that reach the stomach are killed by gastric juice. (pH 2-3). However, organisms embedded in food particles are protected from gastric juice and reach the small intestine. There microorganisms are damaged by various pancreatic enzymes, bile, enzymes in intestinal secretions and GALT system. Normal microbiota of the large intestine is important in preventing the establishment of pathogens. The mucous membranes of the intestinal tract contain paneth cells. These cells produce lysozyme and cryptins (toxic for bacteria).
v) Genitourinary tract
Under normal circumstances, the kidneys, ureters and urinary
bladder of mammals are sterile. Urine within the urinary bladder is also
sterile. In addition to removing microbes by flushing action, urine kills some
bacteria due to its low pH and the presence of urea and other metabolic end
products (uric acid, hippuric acid, indican, fatty acids, mucin, and enzymes).
The acidic environment (pH 3-5) of the vagina is unfavorable to most microbes.
vi) Eye
The conjunctiva is specialized mucus secreting epithelial
membrane that lines the interior surface of each eyelid and the exposed surface
of the eye ball. It is kept moist by the continuous flushing action of tears.
Tears contain large amounts of lysozyme, lactoferrin, and antibody and thus
provide chemical as well as physical protection (Figure 13.18).
B. Chemical mediators
Antimicrobial peptides
They are low molecular weight proteins that exhibit broad spectrum antimicrobial activity toward bacteria.
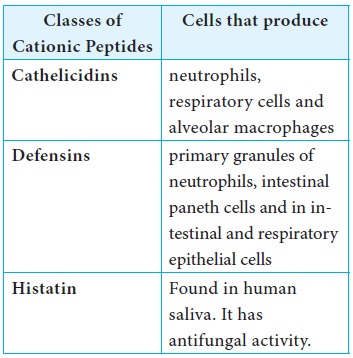
i) Cationic peptides
Cationic peptides are found in humans. There are three generic
classes of cationic peptides that have the ability to damage bacterial plasma
membrane.
ii) Bacteriocins
Bacteriocins are produced by gram negative and gram positive
bacteria. For example, Escherichia coli
synthesize bacteriocins called colicins. Colicins causes cell
lysis.
Cytokines
Cytokines are proteins made by cells that affect the behavior of
other cells. When released from mononuclear phagocytes, they are called monokines. When released from T lymphocytes they are called lymphokines. When released from leukocytes they are called interleukins. Cytokines are required for regulation of both the
nonspecific and specific immune responses. Interferons (IFNS) are a group of
cytokines produced by virus infected cells. Several classes of interferons are
recognized. IFN γ is synthesized by virus infected leukocytes, antigen
stimulated T cells and natural killer cells. IFN α / β is derived from virus
infected fibroblasts. Interferons prevent viral replication and assembly,
thereby limiting viral infection.
Another group of noteworthy cytokines are endogenous pyrogens
which elicit fever in the host. Examples of endogenous pyrogens include interleukin – 1, Interleukin – 6 and
tissue necrosis factor. All are produced by host macrophages in response to
pathogens.
Complement system
The complement system is a part of the immune system, consists
of a series of proteins that interact with one another in a highly regulated
manner, in order to eliminate pathogens. Complements are soluble proteins and
glycoproteins mostly produced by hepatocytes. More than 20 types of complements
are present in serum found circulating normally in human body in inactive forms
(called as zymogens or proenzymes). Complement activation is triggered by an
antibody when it is bound to the antigen. It can also be triggered by some
components of innate immunity. Thus the complement system works in both innate
and acquired immunity.
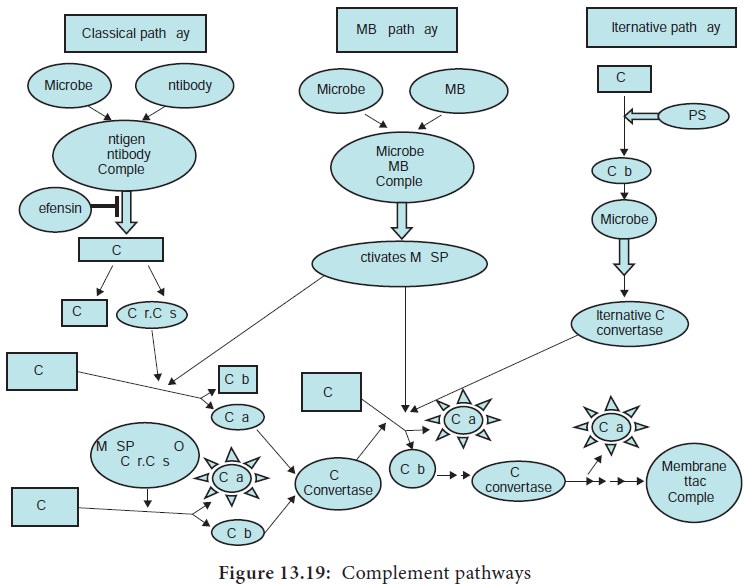
Complement activation and cell lysis
The complement activation occurs via three pathways which are:
1.
Classical pathway
2.
Alternative pathway
3.
Lectin pathway (or mannose binding lectin pathway)
Classical pathway, activated by antigen-antibody reaction,
Alternative pathway, activated on microbial cell surfaces, and Mannose binding
Lectin pathway, activated by a plasma lectin that binds to mannose residues on
microbes (Figure 13.19).
Functions of complements
Some major functions of complements are:
·
Opsonization and phagocytosis
·
Cell lysis
·
Chemotaxis
·
Activation of mast cells and basophils and enhancement of
inflammation
· Production of antibodies
·
Immune clearance and inflamma-tion by attracting macrophages and
neutrophils.
C. Phagocytosis
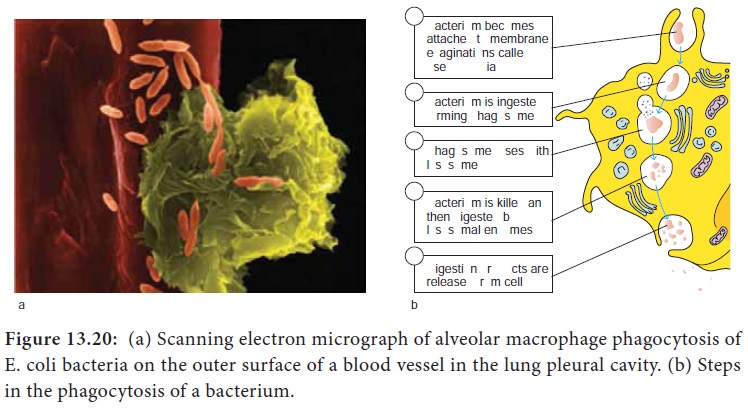
i. Phagocytosis is the ingestion by phagocytic cells of invading
foreign particles such as bacteria. After ingestion, the foreign particle is
entrapped in a phagocytic vacuole (phagosome), which fuses with lysosomes forming the phagolysosome. The lysosomes release their powerful lytic enzymes which digest the particle.
(Figure 13.20). Phagocytosis is conducted by blood monocytes, neutrophils and
tissue macrophages. Phagocytosis may be enhanced by a variety of factors
collectively referred to as opsonins which consist of antibodies and various
serum components of complement.
ii. Phagocytic cells use two basic mechanisms for the
recognition of microorganisms. Opsonin dependent and opsonin independent
iii. Phagocytesuse pathogen recognition receptors to detect pathogen associated molecular patterns on microorganisms. Toll like receptors are a distinct class of pathogen recognition receptors.
D. Inflammation
Tissue damage caused by a wound or by an invading pathogenic
microorganism induces a complex sequence of events collectively known as inflammatory response. Inflammation can
either be acute or chronic. The gross features were described over 2000
years ago and are still known as the cardinal signs of inflammation: redness (rubor), warmth (calor), pain (dolor),
swelling (tumor), and loss of
function (functiolaesa)
The cardinal signs of inflammation reflect the three major
events of an inflammatory response.
1. Vasodilation (an increase in the diameter of blood vessels) of nearby capillaries occurs as the
vessels that carry blood away from the affected area constrict. This results in
engorgement of the capillary network. The engorged capillaries are responsible
for tissue redness (erythema) and an increase in temperature.
2. An increase in capillary permeability facilitates an influx
of fluid and cells from the engorged capillaries into the tissue. The fluid
that accumulates (exudate) has much higher
protein content. Accumulation of exudate contributes to tissue swelling (edema)
3. Influx of phagocytes from the capillaries into the tissues is facilitated by increased capillary permeability. As phagocytic cells accumulate at the site and begin to phagocytoses bacteria, they release lytic enzymes, which can damage nearby healthy cells. The accumulation of dead cells, digested material and fluid forms substances called pus.
Acquired Immunity
Lower animal forms possessso called innate or non-specific
immune mechanisms such as phagocytosis of bacteria by specialized cells. Higher
animals have evolved an adaptive or acquired immune response. This acquired
immune response provides a flexible, specific and more effective reaction to
different infections.
Definition of Acquired (Adaptive) Immunity
Acquired (adaptive)immunity refers to the type of specific
immunity that a host develops after exposure to a suitable antigen.
Important features of acquired immunity
This is the immunity one develops throughout life time. Adaptive
or acquired immunity has four important features namely (1) Memory (2) Specificity diversity and (4)
discrimination between self and non self.
1) Memory
We rarely suffer twice from diseases such as measles, mumps,
chicken pox, whooping cough and so on. The first contact with an infectious
organism clearly imprints some memory so that the body is effectively prepared
to repel any later invasion by that organism.
By following the production of antibody on the first and second
contact with antigen, we can know the basis for the development of immunity.
For example, when we inject a bacterial product such as staphylococcal toxoid
into a rabbit, several days elapse before antibodies can be detected in the
blood. These reach a peak and then fall. If we now allow the animal to rest and
then give a second injection of staphylococcal toxoid, the cause of events is
dramatically altered. Within two to three days the antibody level in the blood
raises steeply to reach much higher values than were observed in the primary
response. This secondary response is characterized by a more rapid and more
abundant production of antibody. This explosive production of antibodies is due
to the tuning up of the antibody forming system to provide a population of
memory cells after first exposure to antigen. The principle of memory is
involved in vaccination.
2) Specificity
The establishment of immunity by one organism does not provide protection against another unrelated organism. After an attack of measles we are immune to further infection but are susceptible to polio or mumps viruses. Thus the body can differentiate specifically between the two organisms.
3) Diversity
The immune system is able to generate an enormous diversity of
molecules such as cellular receptors and soluble proteins, including antibodies
that recognize trillions of different foreign substances.
4) Discrimination between self and nonself
The specific immune system almost responds selectively to non
self and produces specific responses against the stimulus. This is possible
because host cells express a unique protein on their surface, making them as
residents of that host or as self. Thus the introduction of materials lacking
that unique self marker results in their attack by the host.
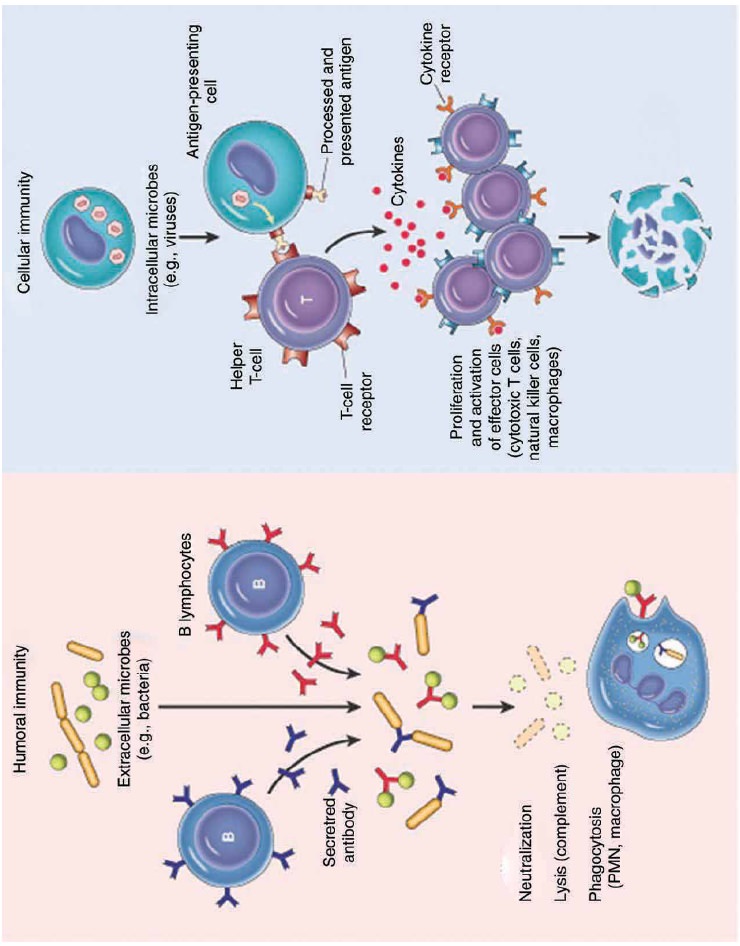
Humoral and Cellular Immunity
Two branches or arms of specific immunity are recognized:
humoral (antibody mediated) immunity and cellular (cell mediated) immunity
(Figure 13.21).
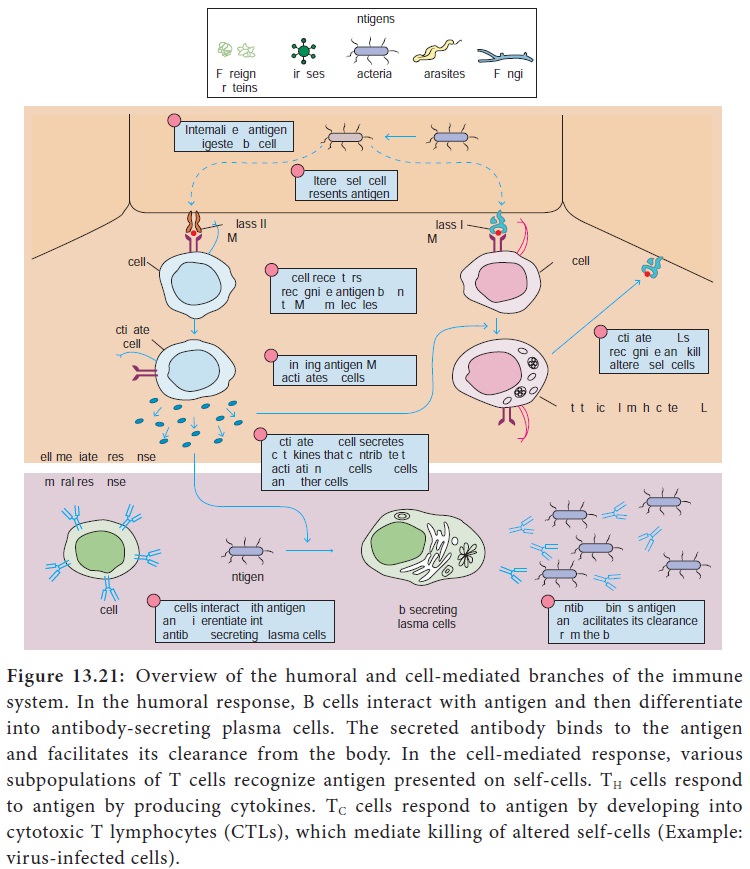
Humoral (antibody mediated) immunity
The antigen specific arm of the humoral immunity consists of the
B cells. Each B cell expresses a unique antigen binding receptor on its
membrane. The B cell receptor (BCR) is membrane bound antibody molecule. When a
naive B cell first encounters the antigen that matches its membrane bound
antibody, the binding of the antigen to the antibody causes the cell to divide
rapidly. Its progeny differentiate into memory B cells and antibody secreting
plasma cells. A single plasma cell can secrete more than 2000 molecules of
antibody per second. Circulating antibodies bind to microorganisms, toxins and
extracellular viruses, neutralizing them or tagging them for destruction by
phagocytes and other mechanisms.
The cellular (cell mediated) immunity consists of the T cells.
Each T cell expresses antigen receptors called T cell receptors (TCRS). Unlike
membrane bound antibody on B cells, which can recognize antigen alone, T cell
receptors can recognize only antigen that is bound to MHC molecules. There are
two major types of MHC molecules. Class I MHC molecules are expressed by all
nucleated cells. Class II MHC molecules are expressed only by antigen
presenting cells such as dendritic cells, macrophages and B cells. When a naive
T cell encounters antigen combined with an MHC molecule on a cell, the T cell
proliferates and differentiates into memory T cells and various effector T
cells (helper T cells, cytotoxic T cells and regulatory T cells). Specific
kinds of T cells directly attack target cells infected with viruses or
parasites, transplanted cells or organs and cancer cells. T cells can induce
target cell suicide (apoptosis), lyse targets cells, or release chemicals
(cytokines) that enhance specific immunity and non specific defences such as
phagocytosis and inflammation.
Types of Specific Immunity
Specific immunity can be acquired by natural means actively through infection or passively through receipt of preformed antibodies as through colostrum. Specific immunity can be acquired by artificial means actively through immunization or passively through receipt of preformed antibodies as with antisera.
Related Topics