Chapter: 11th Microbiology : Chapter 13 : Immunology
Antigen - Antibody Reactions
Antigen - Antibody Reactions
Antigen and antibody combine with each other specifically and in
an observable manner. The exquisite specificity of antigen-antibody
interactions has led to the development of a variety of immunological assays.
These assays can be used to detect the presence of either antibody or antigen.
These assays are also helpful in diagnosing diseases, monitoring epidemiological
surveys and identifying molecules of biological or medical interest.
Antigen-antibody reactions in vitro are known as serological reactions.
Three stages of Antigen – Antibody Reactions
a) Primary stage
The reactions between antigen and antibody occur in three
stages. The primary stage is the initial
interaction between the two
without any visible effects. This reaction is rapid and obeys the general laws
of physical chemistry and thermodynamics. The reaction is reversible. The
combination between antigen and antibody is effected by the weaker
intermolecular forces such as electrostatic forces, hydrogen bonds, Van der
Waals forces and hydrophobic forces. The primary reaction can be detected by
estimating free and bound antigen or antibody separately in the reaction
mixture by a number of physical and chemical methods including the use of
markers such as radioactive isotopes, fluorescent dyes or enzymes.
b) Secondary stage
The primary stage is followed by the secondary stage leading to
demonstrable events such as precipitation, agglutination, lysis of cells,
killing of live antigens, neutralization of motile organisms, complement
fixation and enhancement of phagocytosis.
c) Tertiary stage
Some antigen-antibody reactions occurring in vivo initiate chain
reactions that lead to neutralization or destruction of injurious antigens or
to tissue damage. These are the tertiary reactions and include humoral immunity
against infectious diseases as well as clinical allergy and other immunological
diseases.
General Features of Antigen – Antibody Reactions
Antigen-antibody reactions have the following general
characteristics:
·
The antigen-antibody reaction is specific. An antigen combines
only with its homologous antibody and vice versa. However, the specificity is
not absolute and cross reactions may occur due to antigenic similarity or
relatedness.
·
An entire molecule reacts and not fragments.
·
There is no denaturation of the antigen or the antibody during
the reaction.
·
The combination occurs at the surface.
·
The combination is firm but reversible. The firmness of the
union is influenced by the affinity and avidity of the reaction. Affinity is the strength of binding of one molecule to another at a single site, such as the binding of a monovalent Fab fragment of antibody
to a monovalent antigen. Avidity is the sum total of
the strength of binding of two molecules to one another at multiple sites.
·
Both antigen and antibody participate in the formation of
agglutinates or precipitates.
·
Antigens and antibodies can combine in varying proportions,
unlike chemicals with fixed valence. Both antigens and antibodies are
multivalent. Antibodies are bivalent. Antigens may have valencies up to
hundreds.
Measurement of Antigen and Antibody
Many methods are available for the measurement of antigens and
antibodies participating in the primary, secondary and tertiary reactions.
Measurement may be in terms of mass (Example: mg Nitrogen) or
morecommonlyasunitsortitre.Theantibody titre of a serum is the highest dilution
of the serum which gives an observable reaction with the antigen in the
particular test. The titre of a serum is influenced by the nature and quantity
of the antigen and the type and conditions of the test. Antigens may also be
titrated against sera.
Two important parameters of serological tests are sensitivity
and specificity. Sensitivity refers to the ability
of the test to detect even very minute quantities of antigen and antibody. When
a test is highly sensitive, false negative results will be absent or minimal. Specificity refers to the ability of the test to detect reactions
between homologus antigens and antibodies only. When a test is highly specific,
false positive results will be absent or minimal. Some tests are qualitative
and others are quantitative. The various tests used for detection of antigen
and antibodies are given below:
1.
Precipitation tests
2.
Agglutintion tests
3.
Complement Fixation test
4.
Immunofluorescence
5.
Radio immuno assay
6.
Enzyme linked immuno sorbent assay
7.
Western Blotting technique
8.
Neutralization test
In this section Agglutination and Precipitation reactions will
be described in detail.
1. Precipitation reactions
When a soluble antigen combines with its antibody in the
presence of electrolytes (NaCl) at a suitable temperature and pH, the
antigen-antibody complex, forms an insoluble (visible) precipitate and this
reaction is called precipitation. When instead of
sedimenting, the precipitate remains suspended as floccules, the reaction is
known as flocculation.
Applications of precipitation reactions
The following types of precipitation tests are in common use:
a) Ring test
This test consists of layering the antigen solution over a
column of antiserum in a narrow tube. A visible precipitate forms at the
junction of the two liquids. Examples of ring precipitation test are the C-
reactive protein test, Ascoli’s thermoprecipitin and the grouping of
streptococci by the Lancefield technique.
b) Slide test
When a drop of antigen and a drop of antiserum are placed on a
slide and mixed by shaking, floccules appear. The VDRL test for syphilis is an
example of slide flocculation.
c) Tube test
A quantitative tube flocculation test is used for the
standardization of toxins and toxoids. Serial dilution of the toxin / toxoid is
added to the tube containing a fixed quantity of the antitoxin. The toxin or
toxoid that flocculates optimally with one unit of the antitoxin is defined as
the Lf (Lethal Flocculation) dose.
Precipitation reaction in gels
There are several advantages in allowing precipitation to occur
in a gel rather than in a liquid medium. The reaction is visible as a distinct
band of precipitation, which is stable and can be stained for preservation, if
necessary. Imunodiffusion is usually performed in 1% agarose gel. Different
modifications of the test are available.
·
Single Diffusion in One Dimension (Oudin Procedure)
·
Double Diffusion in One Dimensions (Oakley-Fulthorpe Procedure)
·
Single Diffusion in Two Dimensions (Mancini Procedure)
·
Double Diffusion in Two Dimensions (Ouchterlony Procedure)
Immunoelectrophoresis
Immunoelectrophoresis was devised by Grabar and Williams (1953).
This method consists of two steps. The first step is agarose electrophoresis of
the antigen. Rectangular trough is then cut into the agarose gel parallel to
the direction of the electric field and is filled with the antiserum. By
diffusion, lines of precipitation develop with each of the separated compounds
(Figure 13.28). This method is used to detect normal and abnormal serum
proteins.
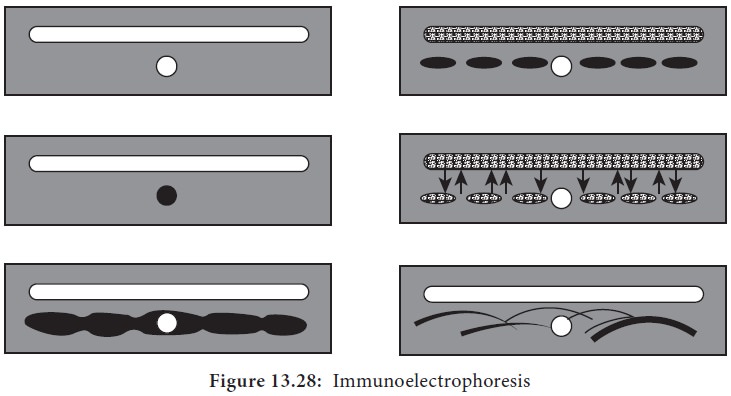
1.
Semisolid agar layered on the glass slide. A well for antigen
and a trough for antiserum cut out of agar.
2.
Antigen well filled with human serum.
3.
Serum separated by electrophoresis.
4.
Antiserum trough filled with antiserum to whole human serum.
5. Serum and antiserum allowed to diffuse into agar.
6.
Precipitin lines form for individual serum proteins
·
Counterimmunoelectrophoresis
·
Rocket Electrophoresis
2. Agglutination reactions
When a particulate antigen is mixed with its antibody in the
presence of electrolytes at a suitable temperature and pH, the particles are
clumped or agglutinated, and the reaction is called agglutination.
Agglutination is more sensitive than precipitation for detection
of antibodies. Agglutination occurs optimally when antigens and antibodies
react in equivalent proportions. Incomplete or monovalent antibodies (having
only one antigen combining site) do not cause agglutination, though they
combine with the antigen. They may act as blocking antibodies inhibiting
agglutination by the complete antibody added subsequently.
Direct agglutination test
In the direct technique, a cell or insoluble
particulate antigen is agglutinated directly by antibody. An example is the
agglutination of group A erythrocytes by anti-A sera.
Indirect (Passive) agglutination test
Passive agglutination refers to
agglu-tination of antigen coated cells or inert particles (bentonite or latex
particles) which are passive carriers of soluble an-tigens. An example is the
latex agglutina-tion for detection of rheumatoid factor. When instead of the
antigen, the antibody is adsorbed to carrier particles in test for estimation
of antigen, this technique is known as reverse passive agglutination.
Hemagglutination inhibition method
The inhibition of agglutination of antigen-coated red blood
cells by homologous antigen is a highly sensitive and specific method for
detecting small quantities of soluble antigen in blood or other tissue fluids.
The principle of this method is that antibody preincubated with soluble
homologous antigen will be inactivated when incubated with antigen coated red
blood cells.
This method is used in the detection of HBs Ag in hepatitis and
in the detection of factor VIII antigen in hemophilia.
Hemagglutination inhibition is also used to detect antibodies
against certain viruses (Arbovirus, Influenza, Measles and Rubella). These
viruses are able to agglutinate red blood cells because they possess
hemagglutinins on their outer surfaces.
Applications of agglutination reactions
a) Slide agglutination
When a drop of the appropriate antiserum is added to a smooth
uniform suspension of a particulate antigen in a drop of saline on a slide,
agglutination takes place. A positive result is indicated by the clumping
together of the particles and the cleaning of the drop. Mixing the antigen and
the antiserum by gently rocking the slide facilitates the reaction.
It is essential to have on the same slide a
controlconsistingoftheantigensuspension in saline, without the antiserum, to
ensure that the antigen is not autoagglutinable. Agglutination is visible to
the naked eye but may sometimes require confirmation under the microscope.
Slide agglutination is a routine test for the identification of many bacterial
isolates from clinical specimens. It is also the method used for blood grouping
and cross matching.
b) Tube agglutination
This is a standard quantitative method for measurement of
antibodies. When a fixed volume of a particulate antigen suspension is added to
an equal volume of serial dilution of an antiserum in test tubes, the agglutination
titre of the serum can be estimated. Widal test done for typhoid and Weil Felix test done for rickettsial infections are examples of Tube agglutination.
Latex agglutination test
Here latex particles are used as passive carriers for adsorbed
soluble antigens. The most widespread application of latex agglutination has
been in the detection of rheumatoid factor. In rheumatoid arthritis, the
patient’s produces rheumatoid factor. Rheumatoid factor is a pentameric IgM
antibody directed against IgG. The test consists of coating latex particles
with IgG and reacting them with the patient serum. Agglutination indicates a
positive test. Latex agglutination tests are also employed in the clinical
laboratory for detection of HBs Ag, ASO (Antistreptolysin O) and CRP
(Carbohydrate Reactive Protein)
Coombs test (antiglobulin test)
This test was devised by Coombs, Mourant and Race (1945) for the
detection of anti-Rh antibodies that do not agglutinate Rh-positive red blood
cells in saline. When sera containing incomplete anti-Rh antibodies are mixed
with Rh- positive red blood cells, the antibody globulin coats the surface of
the red blood cells, though they are not agglutinated. When such red blood
cells coated with antibody globulin are washed free of all unattached protein
and treated with a rabbit antiserum against human gammaglobulin (antiglobulin
or Coombs serum), the cells are agglutinated. This is the principle of the
Coombs test (Figure 13.29).
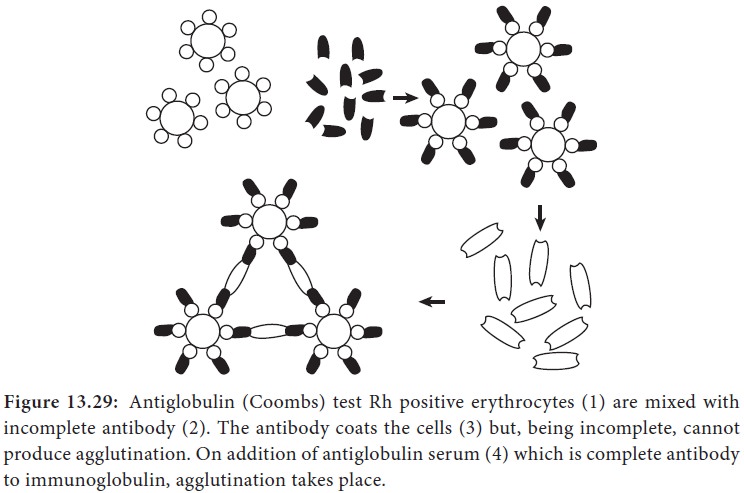
The Coombs test may be of the direct or the indirect type.
Applications of coombs test
1.
Erythrocyte typing in blood banks.
2.
The evaluation of hemolytic disease of the newborn.
3.
The diagnosis of autoimmune hemolytic anemia.
Related Topics