Chapter: Medical Surgical Nursing: Management of Patients With Dermatologic Problems
Malignant Melanoma
MALIGNANT MELANOMA
A
malignant melanoma is a cancerous neoplasm in which atypi-cal melanocytes (ie,
pigment cells) are present in the epidermis and the dermis (and sometimes the
subcutaneous cells). It is the most lethal of all the skin cancers and is
responsible for about 2% of all cancer deaths (Odom et al., 2000).
It
can occur in one of several forms: superficial spreading melanoma,
lentigo-maligna melanoma, nodular melanoma, and acral-lentiginous melanoma.
These types have specific clinical and histologic features as well as different
biologic behaviors. Most melanomas arise from cutaneous epidermal melanocytes,
but some appear in preexisting nevi (ie, moles) in the skin or de-velop in the
uveal tract of the eye. Melanomas occasionally ap-pear simultaneously with
cancer of other organs.
The
worldwide incidence of melanoma doubles every 10 years, a rise that is probably
related to increased recreational sun expo-sure and better methods of early
detection. Peak incidence occurs between ages 20 and 45. The incidence of
melanoma is increas-ing faster than that of almost any other cancer, and the
mortality rate is increasing faster than that of any other cancer except lung
cancer. The estimated number of new cases in 2002 is 53,600 and the number of
deaths is 7400 (American Cancer Society, 2002).
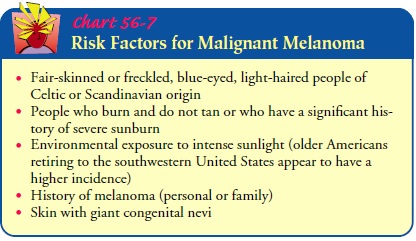
Risk Factors
The
cause of malignant melanoma is unknown, but ultraviolet rays are strongly
suspected, based on indirect evidence such as the increased incidence of
melanoma in countries near the equator and in people younger than age 30 who
have used a tanning bed more than 10 times per year. In general, 1 in 100
Caucasians will get melanoma every year. Up to 10% of melanoma patients are
members of melanoma-prone families who have multiple chang-ing moles (ie,
dysplastic nevi) that are susceptible to malignant transformation. Patients
with dysplastic nevus syndrome have been found to have unusual moles, larger
and more numerous moles, lesions with irregular outlines, and pigmentation
located all over the skin. Microscopic examination of dysplastic moles shows
disordered, faulty growth. Chart 56-7 lists risk factors for malignant
melanoma.
Research has identified a gene that resides on chromosome 9p, the absence of which increases the likelihood that potentially mutagenic DNA damage will escape repair before cell division. The absence of this gene can be identified in melanoma-prone families (Piepkorn, 2000).
Clinical Manifestations
Superficial
spreading melanoma occurs anywhere on the body and is the most common form of
melanoma. It usually affects middle-aged people and occurs most frequently on
the trunk and lower extremities. The lesion tends to be circular, with
irregular outer portions. The margins of the lesion may be flat or elevated and
palpable (Fig. 56-7). This type of melanoma may appear in a combination of colors,
with hues of tan, brown, and black mixed with gray, blue-black, or white.
Sometimes a dull pink rose color can be seen in a small area within the lesion.
LENTIGO-MALIGNA MELANOMAS
Lentigo-maligna
melanomas are slowly evolving, pigmented le-sions that occur on exposed skin
areas, especially the dorsum of the hand, the head, and the neck in elderly
people. Often, the le-sions are present for many years before they are examined
by a physician. They first appear as tan, flat lesions, but in time, they undergo
changes in size and color.
NODULAR MELANOMA
Nodular melanoma is a spherical, blueberry-like nodule with a relatively smooth surface and a relatively uniform, blue-black color (see Fig. 56-7). It may be dome shaped with a smooth sur-face. It may have other shadings of red, gray, or purple. Some-times, nodular melanomas appear as irregularly shaped plaques.
The
patient may describe this as a blood blister that fails to re-solve. A nodular
melanoma invades directly into adjacent dermis (ie, vertical growth) and
therefore has a poorer prognosis.
ACRAL-LENTIGINOUS MELANOMA
Acral-lentiginous
melanoma occurs in areas not excessively ex-posed to sunlight and where hair
follicles are absent. It is found on the palms of the hands, on the soles, in
the nail beds, and in the mucous membranes in dark-skinned people. These
melanomas appear as irregular, pigmented macules that develop nodules. They may
become invasive early.
Assessment and Diagnostic Findings
Biopsy results confirm the diagnosis of melanoma.
An excisional biopsy specimen provides histologic information on the type,
level of invasion, and thickness of the lesion. An excisional biopsy specimen
that includes a 1-cm margin of normal tissue and a por-tion of underlying subcutaneous
fatty tissue is sufficient for staging a melanoma in situ or an early,
noninvasive melanoma. Incisional biopsy should be performed when the suspicious
lesion is too large to be removed safely without extensive scarring. Biopsy
spec-imens obtained by shaving, curettage, or needle aspiration are not
considered reliable histologic proof of disease.
A thorough history and physical examination should
include a meticulous skin examination and palpation of regional lymph nodes
that drain the lesional area. Because melanoma occurs in families, a positive
family history of melanoma is investigated so that first-degree relatives, who
may be at high risk for melanoma, can be evaluated for atypical lesions. After
the diagnosis of mel-anoma has been confirmed, a chest x-ray, complete blood
cell count, liver function tests, and radionuclide or computed to-mography
scans are usually ordered to stage the extent of disease.
Prognosis
The prognosis for long-term (5-year) survival is
considered poor when the lesion is more than 1.5 mm thick or there is regional
lymph node involvement. A person with a thin lesion and no lymph node
involvement has a 3% chance of developing metastases and a 95% chance of
surviving 5 years. If regional lymph nodes are in-volved, there is a 20% to 50%
chance of surviving 5 years. Patients with melanoma on the hand, foot, or scalp
have a better prognosis; those with lesions on the torso have an increased
chance of metas-tases to the bone, liver, lungs, spleen, and central nervous
system. Men and elderly patients also have poor prognoses (Demis, 1998).
Medical Management
Treatment depends on the level of invasion and the
depth of the lesion. Surgical excision is the treatment of choice for small,
su-perficial lesions. Deeper lesions require wide local excision, after which
skin grafting may be needed. Regional lymph node dis-section is commonly
performed to rule out metastasis, although new surgical approaches call for
only sentinel node biopsy. This technique is used to sample the nodes nearest
the tumor and spares the patient the long-term sequelae of extensive removal of
lymph nodes if the sample node is negative (Wagner, 2000).
Immunotherapy has had varied success. Immunotherapy
mod-ifies immune function and other biologic responses to cancer. Several forms
of immunotherapy (eg, bacillus Calmette-Guérin [BCG] vaccine, Corynebacterium parvum, levamisole)
offer en-couraging results. Some investigational therapies include biologic
response modifiers (eg, interferon-alpha, interleukin-2), adaptive
immunotherapy (ie, lymphokine-activated killer cells), and mono-clonal
antibodies directed at melanoma antigens. One of these, proleukin, shows
promise in preventing recurrence of melanoma (Demis, 1998). Under investigation
is the laboratory assay of ty-rosinase, an enzyme believed to be produced only
by melanoma cells (Demis, 1998). Several other studies are attempting to
develop autologous immunization against specific tumor cells. These stud-ies
are still in the early experimental stage but show promise of pro-ducing a
vaccine against melanoma (Piepkorn, 2000).
Current treatments for metastatic melanoma are
largely un-successful, with cure generally impossible. Further surgical
inter-vention may be performed to debulk the tumor or to remove part of the
organ involved (eg, lung, liver, or colon). The rationale for more extensive
surgery, however, is for relief of symptoms, not for cure. Chemotherapy for
metastatic melanoma may be used; however, only a few agents (eg, dacarbazine,
nitrosoureas, cis-platin) have been effective in controlling the disease.
When
the melanoma is located in an extremity, regional per-fusion may be used; the
chemotherapeutic agent is perfused di-rectly into the area that contains the
melanoma. This approach delivers a high concentration of cytotoxic agents while
avoiding systemic, toxic side effects. The limb is perfused for 1 hour with
high concentrations of the medication at temperatures of 39°C to 40°C (102.2°F to 104°F) with a perfusion
pump. Inducing hyperthermia enhances the effect of the chemotherapy so that a
smaller total dose can be used. It is hoped that regional perfusion can control
the metastasis, especially if it is used in combination with surgical excision
of the primary lesion and with regional lymph node dissection.
Related Topics