Plant Tissue Culture - Intellectual Property Right (IPR) | 12th Botany : Chapter 5 : Plant Tissue Culture
Chapter: 12th Botany : Chapter 5 : Plant Tissue Culture
Intellectual Property Right (IPR)
Intellectual Property Right (IPR)
Intellectual property right (IPR) is a category of property that
includes intangible creation of the human intellect, and primarily consists of
copyrights, patents, and trademarks. It also includes other types of rights,
such as trade secrets, publicity rights, moral rights, and rights against
unfair competition.
·
In biotechnology, the transformed microorganisms and plants and technologies
for the production of commercial products are exclusively the property of the
discoverer.
·
The discoverer has the full rights on his property. It should not
be neglected by the others without legal permission.
·
The right of discoverer must be protected and it does by certain
laws framed by a country.
·
The IPR is protected by different ways like patents, copyrights,
trade secrets and trademarks, designs and geographical indications.
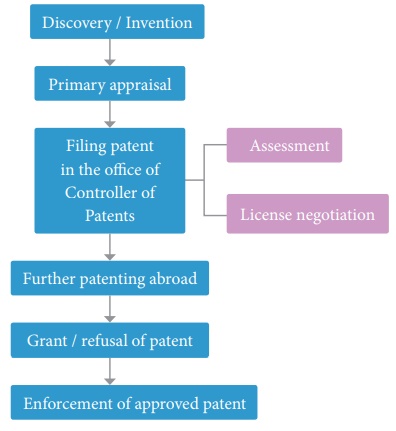
1. Patents
·
It is a special right to the discoverer/inventor that has been
granted by the government through legislation for trading new articles.
·
A patent is a personal property which can be licensed or sold by
the person or organisation just like any other property.
·
Patent terms give the inventor the rights to exclude others from
making, using or selling his invention.
·
It is difficult to keep secret certain inventions and therefore,
guidance should be obtained from a qualified patent attorney.
·
A patent consists of three parts: the grant, specifications and
claims.
·
The grant is filled at the patent office which is not published. It
is a signed document, actually the agreement that grants patent right to the
inventor.
·
The specification and claims are published as a single
document which is made public from the patent office. The specification part is
narrative in which the subject matter of invention is described as how the
invention was carried out.
·
The claim specifically defines the scope of the invention to be
protected by the patent which the others may not practice.
General Steps in Patenting
2. Biosafety and Bioethics
Advances in biotechnology and their applications are mostly
associated with controverisies. This is because the major part of the modern
biotechnology deals with genetic manipulations. ELSI which represents ethical,
legal and social implications of biotechnology broadly
covers the relationship between biotechnology and society with particular
reference to ethical and legal aspects.
Biosafety
Biosafety is the prevention of large-scale loss of biological
integrity, focusing both on ecology and human health. These prevention
mechanisms include conduction of regular reviews of the biosafety in laboratory
settings, as well as strict guidelines to follow. Biosafety is used to protect
from harmful incidents. Many laboratories handling biohazards employ an ongoing
risk management assessment and enforcement process for biosafety. Failures to
follow such protocols can lead to increased risk of exposure to biohazards or
pathogens. Human error and poor techniques contribute to unnecessary exposure
to hazards and compromise the best safeguards set into place for protection.
Potential risks and consideration for safety aspects
·
Pathogenicity of living organisms and viruses - natural and
genetically modified - to infect humans, animals and plants to cause diseases.
·
Toxicity of allergy associated with microbial production.
·
Increasing number of antibiotic resistant pathogenic
microorganisms.
·
Problems associated with the disposal of spent microbial biomass
and purification of effluent from biotechnological process.
·
Safetyaspectsassociatedwithcontamination, infection or mutation of
process strains.
·
Safety aspects associated with the industrial use of micro
organisms containing in vitro recombinants.
Biosafety guidelines are being implemented by:
·
The Institutional Bio-safety Committees (IBSCs) monitor the
research activity at institutional level.
·
The Review Committee on Genetic Manipulation (RCGM) functioning in
the Department of Biotechnology (DBT) monitors the risky research activities in
the laboratories.
·
The Genetic Engineering Approval Committee (GEAC) of Ministry of
Environment and Forest has the power to permit the use of Genetically Modified
Organism (GMO) at commercial level and open field trials of transgenic materials
including agricultural crops, industrial products and health care products.
Bioethics - Ethical, Legal and Social Implications (ELSI)
Bioethics refers to the study of ethical issues emerging from
advances in biology and medicine. It is also a moral discernment as it relates
to medical policy and practice. Bioethicists are concerned with the ethical
questions that arise in the relationships among
life sciences, biotechnology and medicine. It includes the study
of values relating to primary care and other branches of medicine.
The scope of bioethics is directly related to biotechnology,
including cloning, gene therapy, life extension, human genetic engineering,
astroethics life in space, and manipulation of basic biology through altered
DNA, RNA and proteins. These developments in biotechnology will affect future
evolution, and may require new principles, such as biotic ethics, that values
life and its basic biological characters and structures.
The Ethical, Legal, and Social Implications (ELSI) program was
founded in 1990 as an integral part of the Human Genome Project. The mission of
the ELSI program was to identify and address issues raised by genomic research
that would affect individuals, families, and society. A percentage of the Human
Genome Project budget at the National Institutes of Health and the U.S.
Department of Energy was devoted to ELSI research.
Ethical issues in Genomic Research
·
Privacy and fairness in the use of genetic information, including
the potential for genetic discrimination in employment and insurance.
·
The integration of new genetic technologies, such as genetic
testing, into the practice of clinical medicine.
·
Ethical issues surrounding the design and conduct of genetic
research with people, including the process of informed consent.
Genetic Engineering Appraisal Committee (GEAC)
GEAC is an apex body under Ministry of Environment, Forests and
Climate change for regulating manufacturing, use, import, export and storage of
hazardous microbes or genetically modified organisms (GMOs) and cells in the
country. It was established as an apex body to accord approval of activities
involving large scale use of hazardous microorganisms and recombinants in
research and industrial production. The GEAC is also responsible for approval
of proposals relating to release of genetically engineered organisms and
products into the environment including experimental field trials (Biosafety
Research Level trial-I and II known as BRL-I and BRL-II).
Related Topics