Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Pharmacokinetics and Pharmacodynamics of Peptide and Protein Drugs
Immunogenicity and Protein Pharmacokinetics - Pharmacokinetics of Protein Therapeutics
Immunogenicity and Protein Pharmacokinetics
The antigenic potential of protein therapeutics may lead to antibody
formation against the protein therapeutic during chronic therapy. This is
especially of concern if animal-derived proteins are applied in human clinical
studies, but also if human proteins are used in animal studies.
Protein–antibody complexation can not only modulate or even obliterate the biological activity of a protein drug, but may also modify its pharmacoki-netic profile. Elimination clearances of protein drugs, for example, may be either increased or decreased by antibody formation and binding (Fig. 6). An increase in clearance is observed if the protein–antibody complex is eliminated more rapidly than the unbound protein (Rosenblum et al., 1985). This increase may occur when high levels of the protein–antibody complex stimulate its clearance by the reticuloen-dothelial system. In other situations, the serum concentration of a protein can be increased if binding to an antibody slows down its rate of clearance because the protein–antibody complex is eliminated more slowly than the unbound protein (Working and Cossum, 1991). In this case, the complex may act as a depot for the protein and, if the antibody is not neutralizing, a longer duration of the pharmacological action may occur. For example, the clearance of rIFNα2a in cancer patients was increased because of an antibody response. In contrast, human leukocyte IFNβ concentration in rats was decreased 15-fold when circulating antibodies were present. A decrease of clearance in the presence of antibody titers was also detected for t-PA in dogs (Working, 1992).
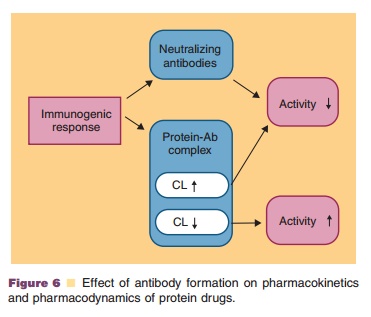
Both an increased and decreased clearance is possible for the same
protein, dependent on the dose level administered. At low doses,
protein–antibody complexes delay clearance because their elimination is slower
than that of the unbound protein. In contrast, at high doses, higher levels of
protein–antibody complex result in the formation of aggregates, which are
cleared more rapidly than the unbound protein.
The enhancement of the clearance of cytokine interleukin-6 (IL-6) via
administration of cocktails of three anti-IL-6 monoclonal antibodies was
suggested as a therapeutic approach in cytokine-dependent diseases like
multiple myeloma, B-cell lymphoma, and rheuma-toid arthritis (Montero-Julian et
al., 1995). The authors could show that, while the binding of one or two
antibodies to the cytokine led to stabilization of the cytokine, simultaneous
binding of three anti-IL-6 anti-bodies to three distinct epitopes induced rapid
uptake of the complex by the liver and thus mediated a rapid elimination of
IL-6 from the central compartment.
The immunogenicity of protein therapeutics is also dependent on the
route of administration. Extravascular injection is known to stimulate
anti-body formation more than IV application, which is most likely caused by
the increased immunogenicity of protein aggregates and precipitates formed at
the injection site.
Related Topics