Chapter: Aquaculture Engineering : Heating and Cooling
Immersion heaters - Aquaculture Engineering
Heaters
Immersion heaters
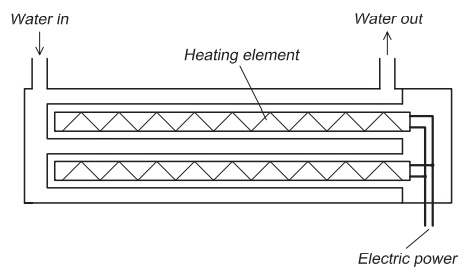
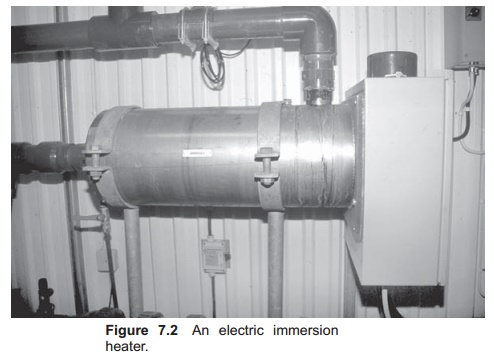
An immersion heater is an electrical element (heat element) placed in the water (Fig. 7.2). Electricity is supplied to the element which heats up. The basic principle is the same as that is used in a heater in a house: the electricity passes through a thin resistant filament where heat develops. It is important that the element is sealed to avoid a short circuit; there-fore it is typically placed in a tube. When water passes the heated element, energy is transferred from the element to the water, the temperature of which increases. Normally a thermostat is attached to the heater so that heat is transferred until the water reaches a set temperature when the electricity supply is automatically/switched off; when the temperature drops below this value, the electricity to the element is automatically switched on. When using an electric heater almost all of the electric energy supplied is transferred to the water. For greatest efficiency when heating requirements is vary, it is an advantage to have several elements in the heater. In addition to a thermostat, the heater is normally equipped with a system to avoid over-heating, for instance when the water flow is reduced or stops. An immersion heater must be correctly installed. There must be no possibility for air pockets inside the heater, because the element may overheat in such places. It is important to choose correct materials for the heating element to avoid corrosion resulting in an electrical short circuit. Materials used are acid-proof steel or stainless steel, depending on the quality and composition of the water to be heated.
The size of heater depends on the energy require-ments. For large water flows or temperature gradients, large heaters are necessary;these consume much elec-tricity as can be inferred from the high cross-sectional area of the wires connected to the heater.
Example
The ambient water temperature is 3┬░C while that in the hatchery is 8┬░C. The hatchery water flow is 60 l/min (1 l/s). Find the size of the heater needed to achieve the required temperature increase.
P= mcpdt
= 1 l/s ├Ś 4.18 kJ/(kg ┬░C) ├Ś (8┬░C ŌłÆ 3┬░C)
= 20.9 kW
The necessary size of the heater is 20.9 kW. The heater is ordered from a supplier and adjusted as required.
Example
On a land-based freshwater production farm the supply of energy necessary, to increase the water temperature to at least 12┬░C throughout the year must be calculated. Water consumption is 2 m3/min.
The incoming freshwater has the following ambient average monthly temperatures:
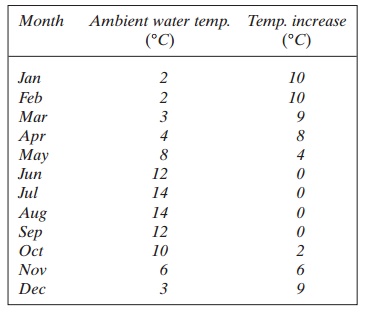
In the months January, February, March, April, May, October, November and December the temperature is so low that it is necessary to heat the water.
Amount of energy required for January is:
P = mcpdt
= 33.3l/s ├Ś 4.18kJ/(kg ┬░C) ├Ś (12┬░C ŌłÆ 2┬░C)
= 1392kW
The energy needed for the whole month is
Q = P ├Ś number of hours
= 1392kW ├Ś 24h ├Ś 31 days
= 1035648kWh
This calculation is repeated for the other months giving the following values:
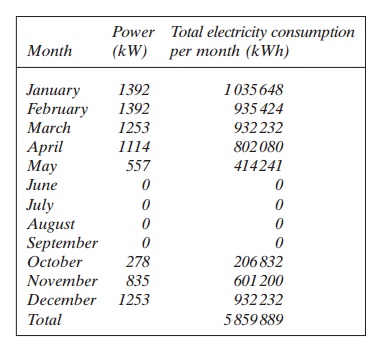
If the price of the electricity is 0.1 Ōé¼/kWh, the yearly cost for heating the water with an immersion heater is
5 859 889 kWh ├Ś 0.1 Ōé¼/kWh = 585 989 Ōé¼
As this example shows, the cost of heating water for fish farming is very high because of the large amount of water. This is also the reason for the great interest in alternative hot water sources and recovery of energy from the outlet water.
Related Topics