Chapter: Aquaculture Engineering : Heating and Cooling
Heating requires energy - Aquaculture Engineering
Heating requires
energy
A supply of energy is needed to heat water. Energy can be
transferred in three different ways: by radiation, by conduction or by
convection. Electromagnetic radiation from the sun or an electric heater, for
example, will be partly absorbed when it falls on a substance and become
internal energy. With conduction, the energy is transferred between solids or
liquids as vibrational energy of the atoms or mole-cules. Additionally, in
materials with a supply of ŌĆśfreeŌĆÖ electrons, e.g. metals, these electrons share
in any energy gain resulting from a temperature rise and their velocities
increase more than those of atoms or molecules, so energy is quickly
transferred to other parts. For example, when the end of an iron bar is heated,
the energy will be transferred to the whole bar and after a short while it will
be hot along its entire length. Convection is heat transfer result-ing from
mixing of substances with different tem-peratures. Convection can be natural or
forced. Natural convection occurs in water as a result of density variation
caused by temperature. Water is of maximum density at 4┬░C. If heated water is
sent in below colder water it will move upwards because it is less dense and
natural convection will take place. Forced convection occurs when a medium such
as water is exposed to an exterior force, for instance a pump or mixer.
The power (P) required
for heating water is pro-portional to the flow and the temperature, and is
given by the equation:
P = mcp dt
where:
m =water flow (kg/s)
cp=specific heat capacity (kJ/(kg ┬░C))
dt= temperature
increase for the water (┬░C).
The specific heat capacity is the amount of energy required to
heat 1 kg water by 1┬░C: cp= 4.18 kJ/ (kg ┬░C)
for freshwater and 4.0 kJ/(kg ┬░C) for seawater. The temperature increase for
the water is the difference between inlet and outlet temperatures.
A continuous power supply is required to heat the flowing water;
the unit of power is normally the kilowatt (kW) and 1 kW = 1 kilojoule (kJ)
per second. Therefore, it can be seen that power is rate of energy transfer.
Example
A freshwater flow of 10 l/min (0.17 l/s) is heated from its original temperature
of 2┬░C to 10┬░C. What is the rate of energy transfer to the water, i.e. the
power supplied?
P =mcpdt
The mass of 1 l of water is 1 kg.
P =0.17 kg/s├Ś4.18 kJ/(kg ┬░C)├Ś(10┬░CŌłÆ2┬░C)
= 5.7 kJ/s
= 5.7 kW
The total amount of energy that has to supplied during a given
period or that has been used during a given period can be calculated from the
power supplied multiplied by the time for which this power is used.
Q =Pt
where:
Q =total amount of energy
(kilowatt-hour, kWh) P =power (kW)
t = time over which
heating takes place (h).
If this is compared to flowing water, the power (kW or kJ/s)
corresponds to water flow rate (l/s), while the total energy consumed
corresponds to the total amount of water which has flowed past a certain point
during a given period of time.
It is the energy consumed (Q)
that is paid for, be it electricity, oil or another energy source. Electricity
is charged per kilowatt-hour, consumed
Example
Calculate the daily cost of heating
the water in the previous example. The price of electricity is 0.1 Ōé¼/kWh.
Q =Pt
=5.7 kW ├Ś 24 h
=136.8 kWh
Therefore the cost is
136.8 kWh ├Ś 0.1 Ōé¼/kWh = 13.68 Ōé¼.
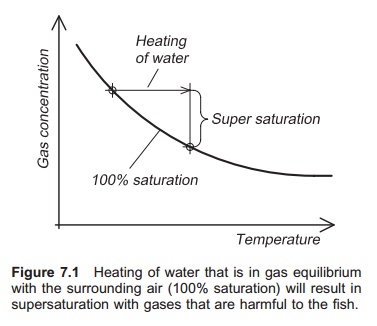
When the water is heated it must always be aerated before it is
used on fish because it may become supersaturated with gas. When the water
temperature increases the amount of dissolved gas is reduced. If there was
equilibrium between the gases in the water and the surrounding air before the
water was heated, the water will be supersatu-rated with these gases afterwards
(Fig. 7.1). Nitro-gen levels will be harmful. It is very important to be aware
of this, because it means that water containing fish must not be heated
directly.
Related Topics