Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Acid-Base Management
Hydrogen Ion Concentration & pH
Hydrogen Ion Concentration & pH
In any aqueous solution, water molecules reversibly
dissociate into hydrogen and hydroxide ions:
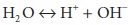
This process is described by the dissociation
constant, KW:
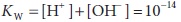
The
concentration of water is omitted from the denominator of this expression
because it does not vary appreciably and is already included in the constant.
Therefore, given [H+] or [OH−], the concentration of the other ion
can be readily calculated.
Example:
If [H+]=10−8nEq/L, then [OH−]=10−14÷ 10−8= 10−6 nEq/L.
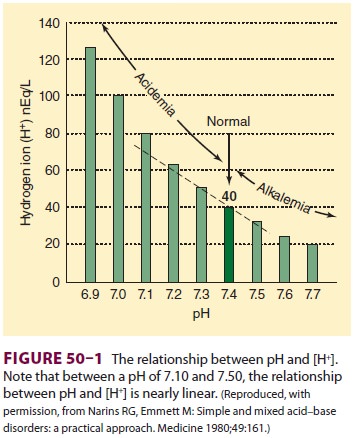
Arterial [H+] is normally 40 nEq/L,
or 40 × 10−9 mol/L. Hydrogen ion concentration is more
commonly expressed as pH, which is defined as the negative logarithm (base 10)
of [H+] (Figure
50–1). Normal arterial pH is therefore –log (40 × 10−9) = 7.40. Hydrogen ion
concentrations between 16 and 160 nEq/L (pH 6.8–7.8) are compatible with life.
Like most
dissociation constants, KW
is affected by changes in temperature. Thus, the electroneutral-ity point for
water occurs at a pH of 7.0 at 25°C, but at about a pH of 6.8 at 37°C; temperature-related changes may be
important during hypothermia .
Because physiological fluids are complex aqueous solutions, other factors that affect the dissociation of water into H + and OH − are the SID, the Pco2, and ATOT.
Related Topics