Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Acid-Base Management
Body Buffers
Compensatory Mechanisms
Physiological responses to changes in [H +] are char-acterized by three phases: (1) immediate chemical buffering,
(2) respiratory compensation (whenever possible), and (3) a slower but more
effective renal compensatory response that may nearly normalize arterial pH
even if the pathological process remains present.
BODY BUFFERS
Physiologically important buffers in humans
include bicarbonate (H2CO3/HCO3−), hemoglobin (HbH/ Hb−), other intracellular
proteins (PrH/Pr−), phos-phates (H2PO4−/HPO42−), and ammonia (NH3/NH4+). The
effectiveness of these buffers in the various fluid compartments is related to
their concentration. Bicarbonate is the most important buffer in the extra-cellular
fluid compartment. Hemoglobin, though restricted inside red blood cells, also
functions as an important buffer in blood. Other proteins probably play a major
role in buffering the intracellular fluid compartment. Phosphate and ammonium
ions are important urinary buffers.
Buffering of the extracellular compartment can also be accomplished by
the exchange of extracel-lular H+ for Na+ and Ca2+ ions from bone and by the exchange
of extracellular H + for
intracellular K +. Acid
loads can demineralize bone and release alka-line compounds (CaCO3 and CaHPO4).
Alkaline loads (NaHCO3) increase the deposition of
carbon-ate in bone.
Buffering by plasma bicarbonate is almost immediate, whereas that due to
interstitial bicar-bonate requires 15–20 min. In contrast, buffering by
intracellular proteins and bone is slower (2–4 h). Up to 50% to 60% of acid
loads may ultimately be buff-ered by bone and intracellular buffers.
The Bicarbonate Buffer
Although in the strictest sense, the bicarbonate buffer consists of H2CO3 and HCO3−, CO2 tension (Pco2) may be
substituted for H2CO3 because:
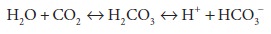
This hydration of CO2 is catalyzed by carbonic anhydrase. If
adjustments are made in the dissocia-tion constant for the bicarbonate buffer
and if the solubility coefficient for CO2 (0.03 mEq/L) is taken into consideration, the Henderson–Hasselbalch
equation for bicarbonate can be written as follows:
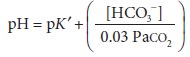
where pK′ = 6.1.
Note that its pK′ is well removed from the nor-mal
arterial pH of 7.40, which means that bicar-bonate would not be expected to be
an efficient extracellular buffer (see above). The bicarbonate system is,
however, important for two reasons: (1) bicarbonate (HCO3−) is present in relatively high
concentrations in extracellular fluid, and (2) more
importantly—Paco2 and plasma [HCO3−] are closely regulated by the lungs
and the kidneys,
respectively. The ability of these two organs to alter the [HCO3−]/Paco2 ratio allows them
to exert important influences on arterial pH.
A
simplified and more practical derivation of the Henderson–Hasselbalch equation
for the bicarbonate buffer is as follows:
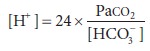
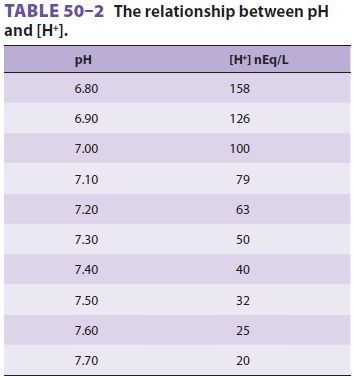
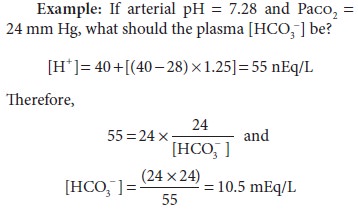
This
equation is very useful clinically because pH can be readily converted to [H+] (Table 50–2). Note that below 7.40, [H+] increases 1.25 nEq/L for each 0.01
decrease in pH; above 7.40, [H+] decreases 0.8 nEq/L for each 0.01
increase in pH.
It should be emphasized that the bicarbonate buffer
is effective against metabolic but notrespiratory
acid–base disturbances. If 3 mEq/L of a strong nonvolatile acid, such as HCl,
is added to extracellular fluid, the following reaction takes place:
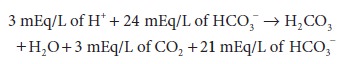
Note that HCO 3− reacts with H + to produce CO2.
Moreover, the CO2 generated is normally elim-inated by the lungs
such that Paco2 does not change. Consequently, [H+] = 24 × 40 ÷ 21 = 45.7 nEq/L, and pH = 7.34. Furthermore, the
decrease in [HCO 3−] reflects the amount of
nonvolatile acid added.
In
contrast, an increase in CO2 tension (vola-tile acid) has a minimal
effect on [HCO3]. If, for example, Paco2 increases from
40 to 80 mm Hg, the dissolved CO 2 increases only from 1.2 mEq/L to
2.2 mEq/L. Moreover, the equilibrium constant for the hydration of CO 2
is such that an increase of this magnitude minimally drives the reaction to the
left:
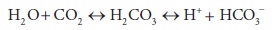
If the
valid assumption is made that [HCO3−] does not appreciably change, then
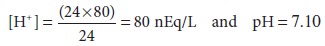
[H+] therefore increases by 40 nEq/L,
and because HCO3− is produced in a 1:1 ratio with H+, [HCO3−] also increases by 40 nEq/L. Thus,
extracel-lular [HCO3−] increases negligibly, from 24 mEq/L
to 24.000040 mEq/L. Therefore, the bicarbonate buffer is not effective against
increases in Paco2, and changes in [HCO3−] do not reflect the severity of a
respiratory acidosis.
Hemoglobin as a Buffer
Hemoglobin is rich in histidine, which is an
effec-tive buffer from pH 5.7 to 7.7 (pKa 6.8). Hemoglobin is the most important
noncarbonic buffer in extra-cellular fluid. Simplistically, hemoglobin may be
thought of as existing in red blood cells in equilibrium as a weak acid (HHb)
and a potassiumsalt (KHb). In contrast to the bicarbonate buf-fer, hemoglobin
is capable of buffering bothcarbonic (CO2) and noncarbonic (nonvolatile) acids:
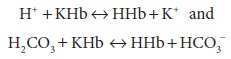
Related Topics