Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Acid-Base Management
Acids & Bases
Acids & Bases
An acid is
usually defined as a chemical species that can act as a proton (H+) donor, whereas a base is a species
that can act as a proton acceptor (Brönsted– Lowry definitions). In
physiological solutions, it is probably better to use Arrhenius’ definitions:
An acid is a compound that contains hydrogen and reacts with water to form
hydrogen ions. A base is a compound that produces hydroxide ions in water.
Using these definitions, the SID becomes important, as other ions in solutions
(cations and anions) will affect the disso-ciation constant for water, and,
therefore, the hydro-gen ion concentration. A strong acid is a substance that readily and almost irreversibly
gives up an H+ and increases [H+], whereas a strong base avidly binds H+ and decreases [H+]. In contrast, weak acids revers-ibly donate H+, whereas weak bases reversibly bind H+; both weak acids and bases tend to
have less of an effect on [H+] (for a given concentration of the
parent compound)than do strong acids and bases. Biological compounds are either
weak acids or weak bases.
For a
solution containing the weak acid HA, where
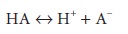
a
dissociation constant, K, can be
defined as follows:
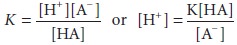
The negative logarithmic form of the latter
equa-tion is called the Henderson–Hasselbalch equation:
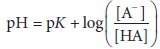
From this
equation, it is apparent that the pH of this solution is related to the ratio
of the dissociated anion to the undissociated acid.
The
problem with this approach is that it is phenomenological—measure the pH and
bicarbon-ate, and then other variables can be manipulated mathematically. This
approach works well with pure water—the concentration of [H +] must equal [OH−]. But physiological solutions are
far more complex. Even in such a complex solution, the [H+] can be predicted using three
variables: the SID, the Pco2, and ATOT.
Related Topics