Chapter: Essentials of Anatomy and Physiology: Respiratory System
Gas Exchange - Respiratory System
Gas Exchange
Ventilation supplies atmospheric air to the alveoli. The next step in the process of respiration is the diffusion of gases between the alveoli and the blood in the pulmonary capillaries. As previously stated, gas exchange between air and blood occurs in the respira-tory membrane of the lungs (see figure 15.8). The major area of gas exchange is in the alveoli, although some takes place in the respira-tory bronchioles and alveolar ducts. Gas exchange between blood and air does not occur in other areas of the respiratory passageways, such as the bronchioles, bronchi, and trachea. The volume of these passageways is therefore called anatomical dead space.
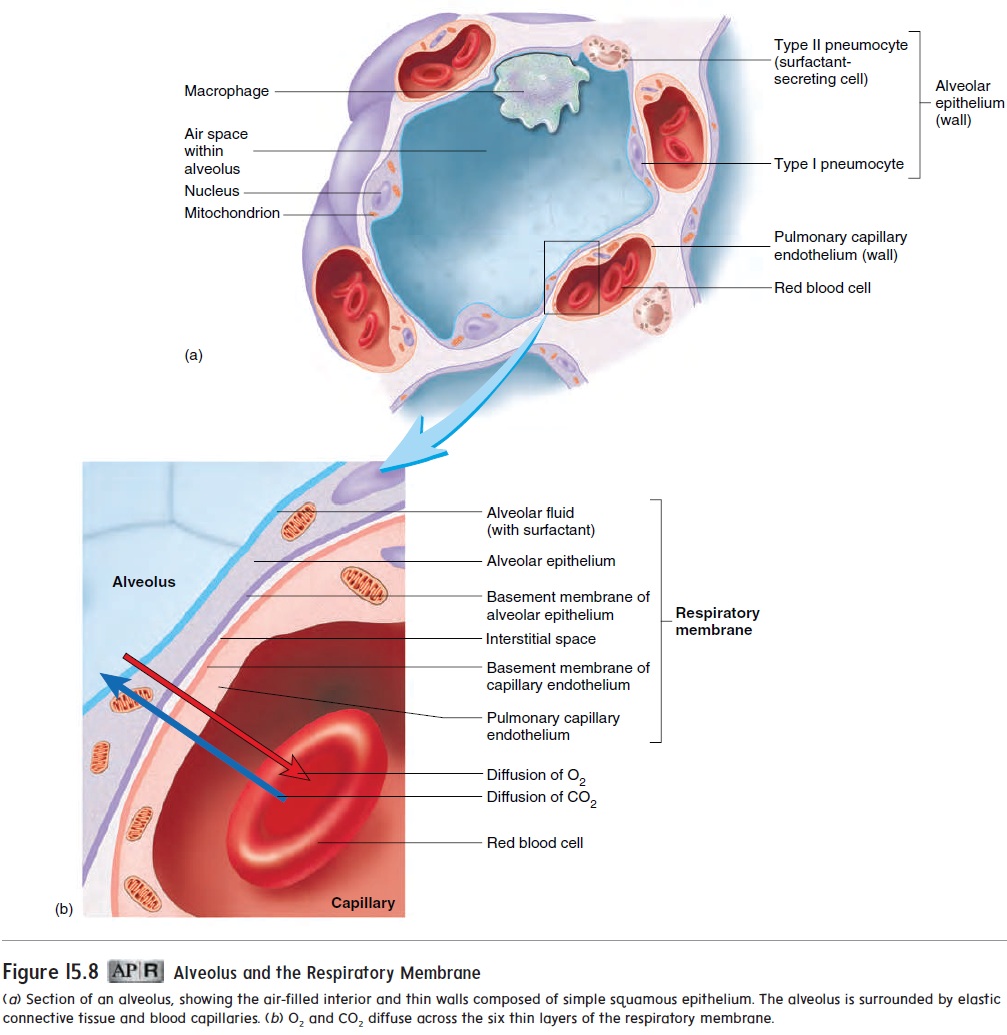
The exchange of gases across the respiratory membrane is influenced by the thickness of the membrane, the total surface area of the respiratory membrane, and the partial pressure of gases across the membrane.
Respiratory Membrane Thickness
The thickness of the respiratory membrane increases during cer-tain respiratory diseases. For example, in patients with pulmonary edema, fluid accumulates in the alveoli, and gases must diffuse through a thicker than normal layer of fluid. If the thickness of the respiratory membrane is doubled or tripled, the rate of gas exchange is markedly decreased. Oxygen exchange is affected before CO2 exchange because O2 diffuses through the respiratory membrane about 20 times less easily than does CO2.
Surface Area
The total surface area of the respiratory membrane is about 70 square meters (m2) in the normal adult, which is approximately the floor area of a 25- × 30-ft room, or roughly the size of a racquetball court (20 × 40 ft). Under resting conditions, a decrease in the sur-face area of the respiratory membrane to one-third or one-fourth of normal can significantly restrict gas exchange. During strenuous exercise, even small decreases in the surface area of the respiratory membrane can adversely affect gas exchange. Possible reasons for having a decreased surface area include the surgical removal of lung tissue, the destruction of lung tissue by cancer, and the degenera-tion of the alveolar walls by emphysema. Collapse of the lung—as occurs in pneumothorax—dramatically reduces the volume of the alveoli, as well as the surface area for gas exchange.
Partial Pressure
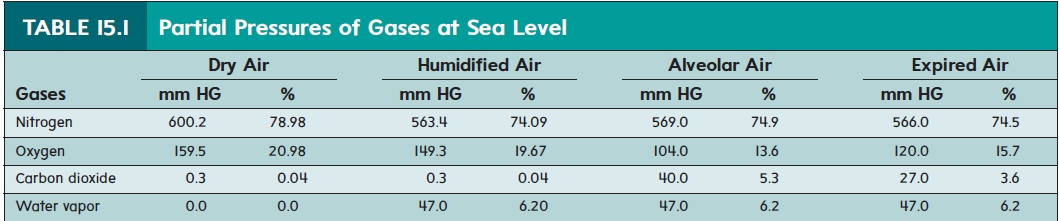
Gas molecules move randomly from higher concentration to lower concentration until an equilibrium is achieved. One measurement of the concentration of gases is partial pressure. The partial pres-sure of a gas is the pressure exerted by a specific gas in a mixtureof gases, such as air. For example, if the total pressure of all the gases in a mixture of gases is 760 millimeters of mercury (mm Hg), which is the atmospheric pressure at sea level, and 21% of themixture is made up of O , the partial pressure for O is 160 mm Hg(0.21*760 mm Hg=160 mm Hg). If the composition of air is0.04% CO at sea level, the partial pressure for CO is 0.3 mm Hg(0.0004 *760= 0.3 mm Hg) (table 15.1). It is traditional to designate the partial pressure of individual gases in a mixture with a capital P followed by the symbol for the gas. Thus, the partial pressure of O2 is Po2, and that of CO2 is Pco2.
When air is in contact with a liquid, gases in the air dissolve in the liquid. The gases dissolve until the partial pressure of each gas in the liquid is equal to the partial pressure of that gas in the air. Gases in a liquid, like gases in air, diffuse from areas of higher partial pres-sure toward areas of lower partial pressure, until the partial pressures of the gases are equal throughout the liquid. In other words, gases diffusedown their pressure gradient; when atmospheric air comes into contact with the water-based fluid in the lungs, CO2 and O2 dis-solve in the fluid and each diffuses down its pressure gradient.
Diffusion of Gases in the Lungs
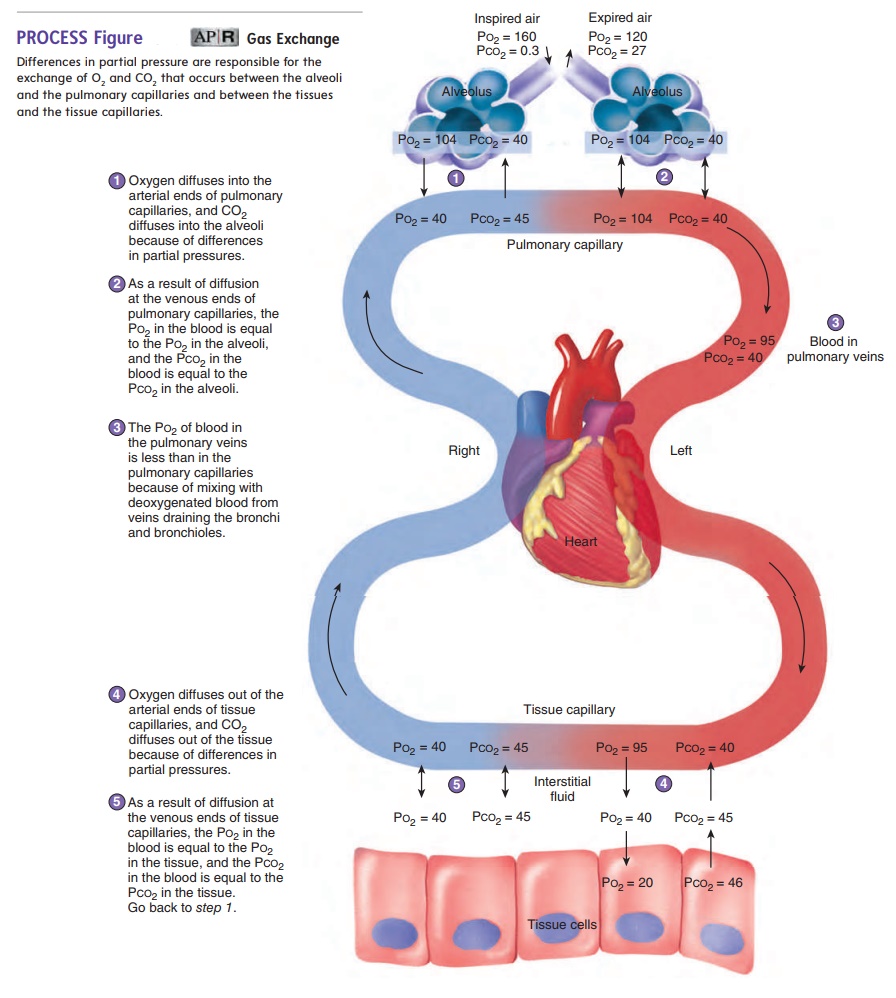
The cells of the body use O2 and produce CO 2. Thus, blood return-ing from tissues and entering the lungs has a decreased Po2 and an increased Pco2 compared to alveolar air (figure 15.13). Oxygen diffuses from the alveoli into the pulmonary capillaries because the Po2 in the alveoli is greater than that in the pulmonary capillar-ies. In contrast, CO2 diffuses from the pulmonary capillaries into the alveoli because the Pco2 is greater in the pulmonary capillaries than in the alveoli (figure 15.13, step 1) .
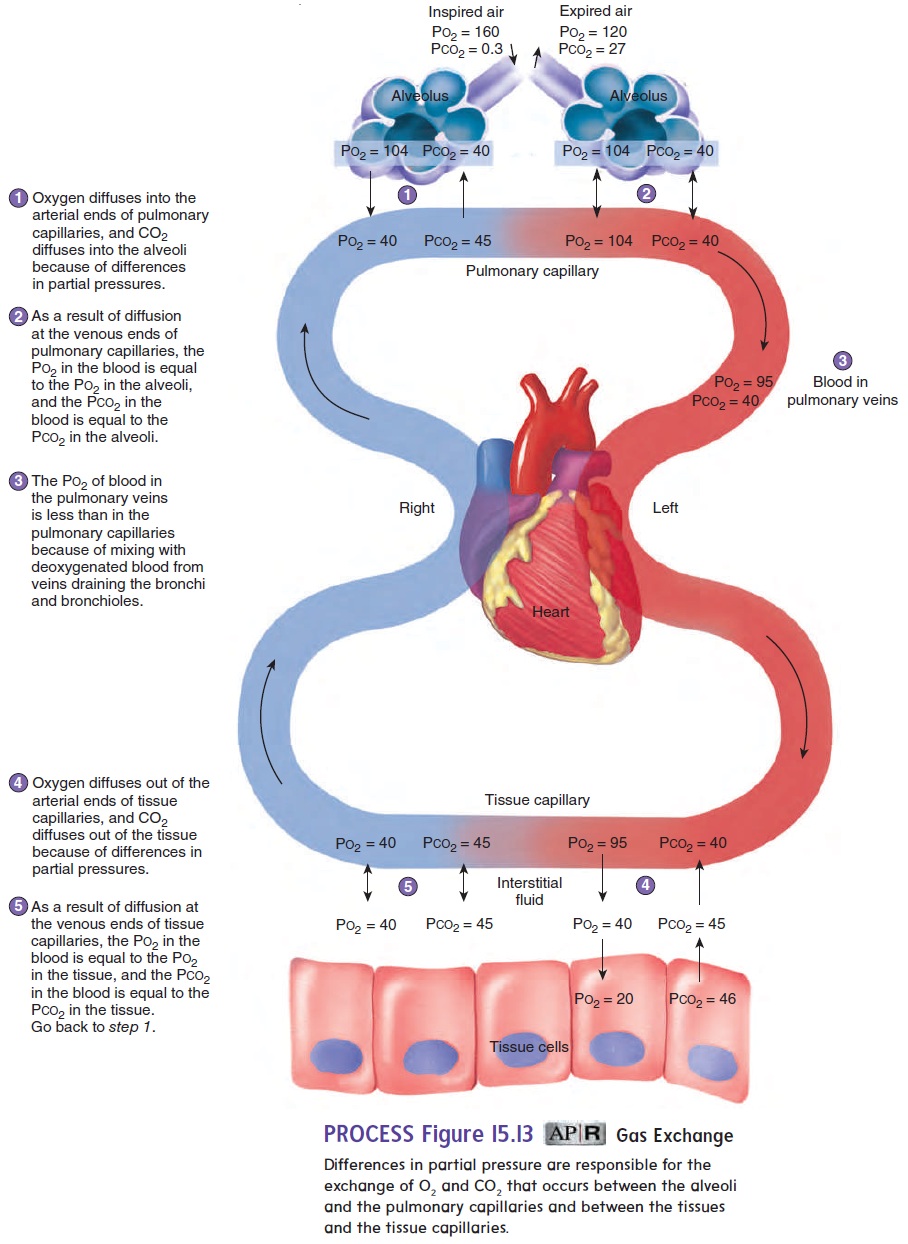
When blood enters a pulmonary capillary, the Po2 and Pco2 in the capillary are different from the Po2 and Pco2 in the alveolus. By the time blood flows through the first third of the pulmonary capillary, an equilibrium is achieved, and the Po2 and Pco2 in the capillary are the same as in the alveolus. Thus, in the lungs, the blood gains O2 and loses CO2 (figure 15.13, step 2).
During breathing, atmospheric air mixes with alveolar air. The air entering and leaving the alveoli keeps the Po2 higher in the alveoli than in the ulmonary capillaries. Increasing the breath-ing rate makes the Po2 even higher in the alveoli than it is during slow breathing.
During labored breathing, the rate of O2 diffusion into the pulmonary capillaries increases because the difference in partial pressure between the alveoli and the pulmonary capillaries has increased. There is a slight decrease in Po2 in the pulmonary veins due to mixing with deoxygenated blood from veins draining the bronchi and bronchioles; however, the Po2 in the blood is still higher than that in the tissues (figure 15.13, step 3).
Increasing the rate of breathing also makes the Pco2 lower in the alveoli than it is during normal, quiet breathing. Because the alveolar Pco2 decreases, the difference in partial pressure between the alveoli and the pulmonary capillaries increases, which increas-es the rate of CO2 diffusion from the pulmonary capillaries into the alveoli.
Diffusion of Gases in the Tissues
Blood flows from the lungs through the left side of the heart to the tissue capillaries. Figure 15.13 illustrates the partial pressure differ-ences for O2 and CO2 across the wall of a tissue capillary. Oxygen diffuses from the capillary into the interstitial fluid because the Po2 is lower in the interstitial fluid than in the capillary. Oxygen diffuses from the interstitial fluid into cells, in which the Po2 is less than in the interstitial fluid (figure 15.13, step 4). Within the cells, O2 is used in cellular respiration. There is a constant difference in Po2 between the tissue capillaries and the cells because the cells con-tinuously use O2. There is also a constant diffusion gradient for CO2 from the cells. Carbon dioxide therefore diffuses from cells into the interstitial fluid and from the interstitial fluid into the tissue capil-laries, and an equilibrium between the blood and tissues is achieved (figure 15.13, step 5).
Related Topics