Chapter: Clinical Anesthesiology: Anesthetic Management: Cardiovascular Physiology & Anesthesia
Effects of Anesthetic Agents on Heart
EFFECTS OF ANESTHETIC AGENTS
Most volatile anesthetic agents are
coronary vasodi-lators. Their effect on coronary blood flow is vari-able
because of their direct vasodilating properties, reduction of myocardial
metabolic requirements (and secondary decrease due to autoregulation), and
effects on arterial blood pressure. The mecha-nism is not clear, and these
effects are unlikely to have any clinical importance. Halothane and iso-flurane
seem to have the greatest effect; the former primarily affects large coronary
vessels, whereas the latter affects mostly smaller vessels. Vasodilation due to
desflurane seems to be primarily autonomi-cally mediated, whereas sevoflurane
seems to lack coronary vasodilating properties. Dose-dependent abolition of
autoregulation may be greatest with isoflurane.
Volatile agents exert beneficial effects
in experi-mental myocardial ischemia and infarction. They reduce myocardial
oxygen requirements and protect against reperfusion injury; these effects are
mediated by activation of ATP-sensitive K + (KATP) channels. Some evidence also suggests
that volatile anesthetics enhance recovery of the “stunned” myocardium
(hypocontractile, but recoverable, myocardium after ischemia). Moreover,
although volatile anesthetics decrease myocardial contractility, they can be
poten-tially beneficial in patients with heart failure because most of them
decrease preload and afterload.
The Pathophysiology of Heart Failure
Systolic heart failure occurs when the
heart is unable to pump a sufficient amount of blood to meet the body’s
metabolic requirements. Clinical manifesta-tions usually reflect the effects of
the low cardiac output on tissues (eg, fatigue, dyspnea, oxygen debt,
acidosis), the damming-up of blood behind the failing ventricle (dependent
edema or pulmo-nary venous congestion), or both. The left ventricle is most
commonly the primary cause, often with secondary involvement of the right
ventricle. Iso-lated right ventricular failure can occur in the set-ting of
advanced disease of the lung parenchyma or pulmonary vasculature. Left
ventricular failure is most commonly the result of myocardial dysfunc-tion,
usually from coronary artery disease, but may also be the result of viral
disease, toxins, untreated hypertension, valvular dysfunction, arrhythmias, or
pericardial disease.
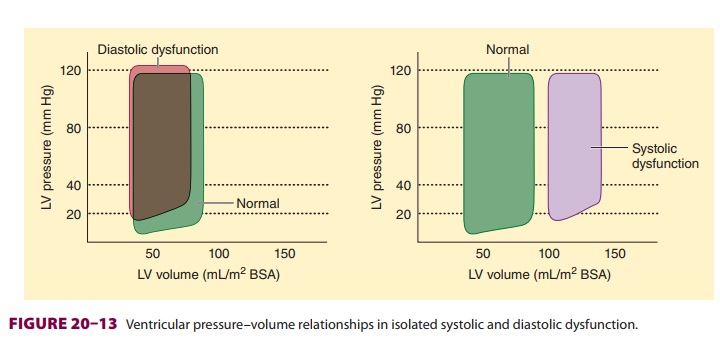
Diastolic dysfunction can be present in
the absence of signs or symptoms of heart failure. Symp-toms arising from
diastolic dysfunction are the result of atrial hypertension (Figure 20–13).
Failure of the heart to relax during diastole leads to elevated left
ventricular end-diastolic pressure, which is trans-mitted to the left atrium
and pulmonary vasculature. Common causes of diastolic dysfunction include
hypertension, coronary artery disease, hypertrophic cardiomyopathy, valvular
heart disease, and peri-cardial disease. Although diastolic dysfunction can
occasionally cause symptoms of heart failure, even in the presence of normal
systolic function (normal left ventricular ejection fraction), it nearly always
occurs in association with systolic dysfunction in patients with heart failure.
Diastolic dysfunction is diagnosed
echocar-diographically. Placing the pulse wave Doppler sample gate at the tips
of the mitral valve during
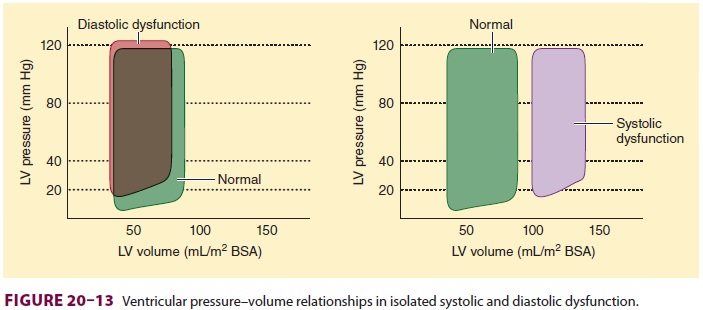
left ventricular filling will produce
the characteris-tic diastolic flow pattern (Figure 20–9). In patients with
normal diastolic function, the ratio between the peak velocities of the early
(E) and the atrial (A) waves is from 0.8 to 2. In the early stages of diastolic
dysfunction, the primary abnormality is impaired relaxation. When left
ventricular relaxation is delayed, the initial pressure gradient between the
left atrium and the left ventricle is reduced, result-ing in a decline in early
filling, and, consequently, a reduced peak E wave velocity. The A wave
veloc-ity is increased relative to the E wave, and the E/A ratio is reduced. As
diastolic dysfunction advances, the left atrial pressure increases, restoring
the gradi-ent between the left atrium and left ventricle with an apparent
restoration of the normal E/A ratio. This pattern is characterized as
“pseudonormal-ized.” Using the E/A ratio alone cannot distinguish between a
normal and pseudonormalized pattern of diastolic inflow. As diastolic dysfunction
worsens further, a restrictive pattern is obtained. In this sce-nario, the left
ventricle is so stiff that pressure builds in the left atrium, resulting in a
dramatic peak of early filling and a prominent, tall, narrow E wave. Because
the ventricle is so poorly compliant, the atrial contraction contributes little
to filling, result-ing in a diminished A wave and an E/A ratio greater than
2:1.Doppler patterns of pulmonary venous flow have been used to distinguish
between a pseudonormalized and normal E/A ratio. Currently, most
echocardiographers use tissue Doppler to examine the movement of the lateral
annulus of the mitral valve during ventricular filling (Figure 20–9). Tissue
Doppler allows the echocardiographer to determine both the velocity and the direction
of the movement of the heart. During systole, the heart contracts toward the
apex, away from a TEE transducer in the esophagus. This motion produces the s’
wave of systole. During early and late diastolic filling, the heart moves
toward the transducer pro-ducing the e’ and a’ waves. Like the inflow patterns
achieved with pulse wave Doppler, characteristic patterns of diastolic
dysfunction are reflected in the tissue Doppler trace. An e’ wave less than 8
cm/sec is consistent with diastolic dysfunction. Of note, the tissue Doppler
trace does not produce a pseudonor-malized pattern permitting the
echocardiographer to readily distinguish between normal and abnor-mal diastolic
function.
Cardiac output may be reduced at rest with heart failure, but the key point is
that the heart is incapable of appropriately increasing cardiac output and oxygen delivery in response to
demand. Inad-equate oxygen delivery to tissues is reflected by a low mixed
venous oxygen tension and an increase in the arteriovenous oxygen content
difference. In com-pensated heart failure, the arteriovenous difference may be
normal at rest, but it rapidly widens during stress or exercise.
Heart failure is less commonly
associated with an elevated cardiac output. This form of heart fail-ure is most
often seen with sepsis, thyrotoxicosis, and other hypermetabolic states, which
are typically associated with a low SVR.
Related Topics