Chapter: Medicine Study Notes : Gastro-Intestinal
Colorectal Cancer
Colorectal Cancer
Presentation
·
Change in shape of stools
significant (e.g. pencil shaped)
·
Rectal bleeding/mucus
·
Persistent changes in bowel habit
·
Tenesmus: constant feeling of
need to defacate, even after passing stool
·
Weight loss
·
Anaemia
·
Abdominal or rectal mass in late
presentation
·
Hepatomegaly (from secondaries)
·
By 75 years, 1 in 20 males and 1
in 25 females (Victoria)
Risk Factors
·
Environmental factors: diet,
lifestyle, smoking
·
Family history
·
Premalignant lesions
Family History and Colorectal Cancer
· Average risk = up to two 1st or 2nd degree relatives with bowel cancer at 55 or older (as long as on different sides of family). 98% of population
· Moderate risk = either one 1st degree relative diagnosed before 55 years, or two 1st or 2nd degree relatives on one side of family diagnosed at any age. 1% population. Refer for colonoscopy every 5 years from age 50 or 10 years younger than earliest diagnosis in family
·
High risk = more than above,
including FAP or identified high-risk mutation in near relative. <1% of
population. Suggest genetic testing. Referral to plan appropriate surveillance
·
Incidence has been declining since
1950‟s (5.8% decrease in men, 12.9% decrease in women)
·
People may not know family
history: can they get death certificates of relatives
Polyps
·
= Any lesion which protrudes
above the level of the surrounding mucosa
·
Hyperplastic polyps
o 90% of all polyps, measure < 0.5 cm, mainly in rectosigmoid
o Benign, no premalignant potential
o “Dew drop” appearance, on top of mucosal fold
o Sessile (no stalk), often multiple, very common
o Microscopically: elongated crypts, goblet and absorptive cells, excess
mucin
·
Adenomatous polyps
o 10% of polyps
o Neoplasms: precursors of most colonic carcinomas. Normally removed as don‟t know which will become invasive
o Malignancy related to size: <1.5 cm only 1% contains a carcinoma, > 1.5 cm 10% will contain a carcinoma-in-situ. As long as it‟s confined to the mucosa, there is no metastatic potential. If submucosal invasion then segmental resection
o Macroscopically: stalk (ie pedunculated)
o Microscopically: neoplastic glands, hyperchromatic, etc
o Tubular adenomas: Most common (75%), usually pedunculated, most common in left colon, M > F, 50% are solitary, continued colonoscopic follow-up necessary. Head has closely packed tubules/glands lined by non-differentiated neoplastic columnar cells. Stalk has normal colonic mucosa
o Villous adenomas: Papillary projections, larger, more likely to harbour
carcinoma, sessile, 10 – 15% of adenomatous polyps, mainly
rectosigmoid
o Tubulovillous adenomas: contain 25 – 75% of villous component. May secrete lots of mucous
·
Juvenile polyps:
o Left side of large bowel of kids
o Cause rectal bleeding
o Grossly look similar to adenomas
o Microscopically not neoplastic. Cystically dilated mucous glands,
inflammation of lamina propria, maybe ulceration
·
Peutz-Jeghers Syndrome: Polyp
containing mucin filled cysts and smooth muscle in the lamina propria. No
malignant potential. Maybe pigmentation in the mouth
·
Polyposis syndromes:
o Familial Adenomatous Polyposis (FAP): 0.5% of all colorectal cancers. Autosomal dominant,
o antioncogene mutation of APC gene. APC gene: 1 in 10,000 have mutation ® 100s of adenomatous polyps appearing in 2nd or 3rd decade. Will develop carcinoma Þ prophylactic colectomy. APC gene also mutated in sporadic cancer
o Hereditary Non-polyposis Colon Cancer (HNPCC): Aka Lynch Syndrome. 5% of
non-FAP colorectal cancers. Patients often young with multiple tumours. 1 – 5 %
of all CR cancer. Autosomal dominant – defect of DNA mismatch repair genes –
MMR gene (mismatch repair) - 1 in 1,000 have mutation
·
Gardener‟s Syndrome: colonic
polyposis, epidermoid cysts (skin), osteoid osteomas (benign bone tumours).
High risk of carcinoma
·
Turcot‟s Syndrome: colon polyps +
brain tumours
Adenocarcinomas of the Colon
·
Epidemiology:
o In US, 2nd only to lung cancer in cancer deaths. Much lower in third world (Þ
environmental factors)
o Peak incidence in 7th decade (ie old), except APC and UC
o 70% in recto-sigmoid colon, rest all the way back to caecum
o M:F is 2:1 for rectal, equal for right sided
·
Aetiology:
o Adenomatous polyps (esp villous)
o Ulcerative colitis
o Familial multiple polyposis
o Family History
o Environmental factors: High incidence in Europe/North America, low in Asia/Africa. Urban > rural
o Diet: High fat and low fibre (slower transit ® ?exposure
to carcinogens)
·
Pathogenesis: increasing loss of
heterozygosity in genes involved in DNA repair, tumour suppression and oncogene
activation. Either through turnover due to mucosal damage ® risk of gene match failure or
directly genotoxic mechanism
·
Presentation:
o Left sided: annular encircling ® napkin ring or apple core constriction. Signs of obstruction. Poorer prognosis despite earlier detection due to invasion
o Right sided: large fungating or sessile masses, necrotic areas, occult bleeding, anaemia, weight loss
o May produce mucin, ulceration ® blood loss
o Doubling time of about 2 years
·
Macroscopic description: Early:
may still appear to be a polyp or sessile. Later: obliterate precursor adenoma
·
Microscopic appearance: Most are
moderately differentiated, irregular glands with pleomorphic cells, usually
lack mucin production. Mucinous carcinomas (10 – 15%) have pools of mucin,
cleaves through tissue aiding spread (worse prognosis)
·
Variants: Adenosquamous
carcinoma, small cell undifferentiated (rare), Ulcerative colitis ® poorly
differentiated colitis
Other large bowel tumours (Gastro-intestinal stromal tumours: GIST)
· Carcinoid tumours: most common in appendix and stomach.
·
Lymphomas: Non-Hodgkin's. Eg MALT
·
Mesenchymal tumours (eg
leiomyomas): much less common
Diagnosis
·
Rectal exam
·
FOBT: sensitivity 50% and low
specificity
·
Sigmoidoscopy picks up 40% (&
take biopsy).
·
Colonoscopy: expensive, miss rate
for cancer 2-3%
·
Double contrast barium enema:
cheaper, miss rate for cancer 10 – 15%
·
Check for dissemination: LFT,
Abdominal CT, CXR (25% have metastatic disease at presentation)
Differential
·
Diverticular disease,
Inflammatory Bowel Disease, Irritable Bowel Syndrome, Rectal ulcer
Treatment of Colorectal Cancer
·
No role for radiotherapy in colon
·
Adjuvant chemotherapy:
o Improves 5 year survival over surgery alone from 50 to 60/65% (but can‟t predict who will benefit)
o Quality
of life (side effects of cancer are pretty severe, chemo reduces these)
o Given after surgery
o Six months of 5FU
o Currently given to:
§ Dukes C: All patients (C1 = Muscularis propria + lymph node, C2 = serosa + lymph node)
§ Dukes B2 (serosa): High risk groups, perforation, invasion of adjacent
organs, diploid tumours
·
Rectal cancer:
o No serosa around rectum – cancer infiltrates straight into fat – harder to get clear resection margins
o Radiation in rectal cancer good: but ® impaired function and may irradiate small bowel ® fibrosis. Try and predict who needs irradiation and do it pre-operatively
·
Palliation: hospice +
chemotherapy better quality of life than hospice alone
Prognosis of Colorectal Cancer
·
Invade into serosal fat,
metastasise to regional lymph nodes then to liver and lungs. Rarely
intraperitoneal spread
·
Complications: Obstruction,
perforation, haemorrhage, fistulas
·
Prognosis mainly related to stage
(how far it‟s spread), to a lesser extent the grade and location
·
Pre-operative staging: ultrasound
of liver, Xray of lungs
·
Duke‟s Post-operative staging:
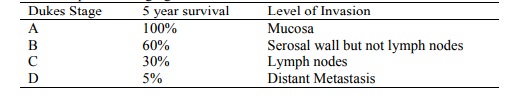
·
Wgtn Hospital uses APC staging
(Australasian Pathologists): minor differences to Dukes
·
5 year survival = cured –
unlikely to relapse after that
Follow-up
·
Colonoscopy (e.g. initially rest
of colon for 2nd primary, then every 3 years)
·
Monitor tumour marker CEA
(colonic embryonic antigen). Also raised in a variety of other tumours &
benign cancers. Not sensitive for early cancers (4% of Duke‟s A). Neither
sensitive nor specific
Related Topics