Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
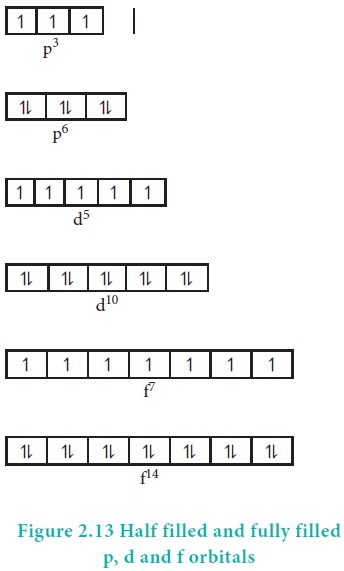
Stability of half filled and completely filled orbitals
Stability of half filled and completely filled orbitals:
The exactly half filled and completely filled orbitals have greater stability than other partially filled configurations in degenerate orbitals. This can be explained on the basis of symmetry and exchange energy. For example chromium has the electronic configuration of [Ar]3d5 4s1 and not [Ar]3d4 4s2 due to the symmetrical distribution and exchange energies of d electrons.
Symmetrical distribution of electron:
Symmetry leads to stability. The half filled and fully filled configurations have symmetrical distribution of electrons (Figure 2.13) and hence they are more stable than the unsymmetrical configurations.
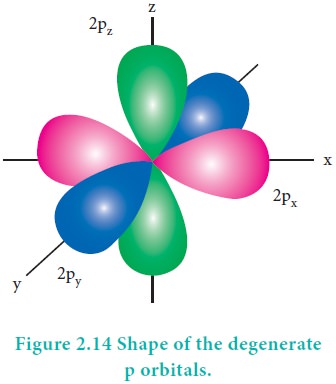
The degenerate orbitals such as px, py and pz have equal energies and their orientation in space are different as shown in Figure 2.14. Due to this symmetrical distribution, the shielding of one electron on the other is relatively small and hence the electrons are attracted more strongly by the nucleus and it increases the stability.
Exchange energy:
If two or more electrons with the samespinarepresentindegenerateorbitals, there is a possibility for exchanging their positions. During exchange process the energy is released and the released energy is called exchange energy. If more number of exchanges are possible, more exchange energy is released. More number of exchanges are possible only in case of half filled and fully filled configurations.
For example, in chromium the electronic configuration is [Ar]3d5 4s1. The 3d orbital is half filled and there are ten possible exchanges as shown in Figure 2.15. On the other hand only six exchanges are possible for [Ar]3d4 4s2 configuration. Hence, exchange energy for the half filled configuration is more. This increases the stability of half filled 3d orbitals.
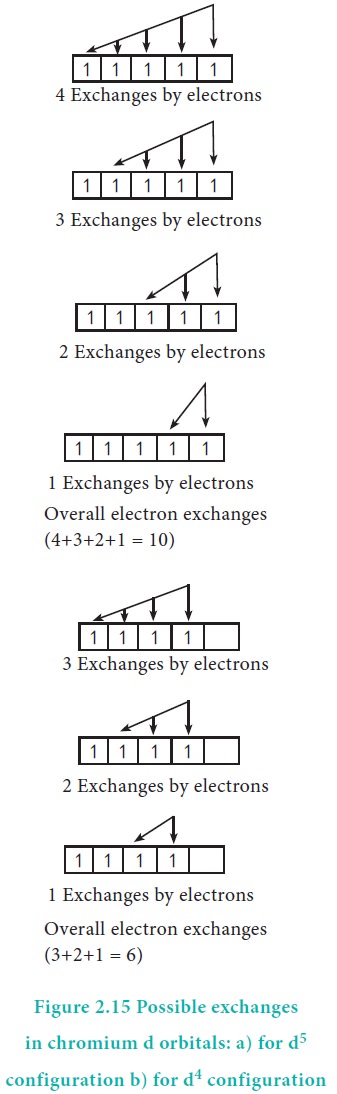
The exchange energy is the basis for Hund's rule, which allows maximum multiplicity, that is electron pairing is possible only when all the degenerate orbitals contain one electron each.
Related Topics