Science - Separation of Mixtures | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
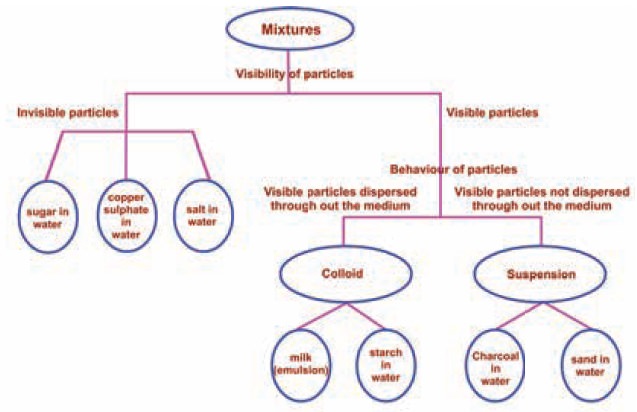
Separation of Mixtures
Separation of Mixtures
At the end of the lesson you will be able to
·
define
key terms such
as solute, solvent, solution, filtration, filtrate, distillation,
distillate, centrifugation, and chromatography
·
analyse and select appropriate methods
for separating a given mixture, based on certain difference in physical
properties
·
describe appropriate methods of separating a given mixture
·
perform simple experiments involving separation of mixtures
·
identify and assemble the suitable set of apparatus used for separating the components of a given mixture
·
explain the basic principles involved
in filtration, centrifugation, distillation and chromatography
·
gather information about the industrial
applications of the different techniques of separation

1. Introduction
A mixture as you know contains more than one
substance in which the components can either be elements or compounds or both.
We separate the components of a mixture very often as they contain useful
substances mixed with harmful or unwanted substances which have to be removed.
The choice of a particular method to separate components of a mixture will
depend on the properties of the components of the mixture as well as their
physical states.
2. Separation of solid – liquid mixtures
Before we talk about the separation methods let us
recall briefly some aspects of solubility of solid and liquid. When a solid is
added to a liquid, either the solid will dissolve in the liquid or not.
·
When the solid dissolves in the liquid, it is said to be soluble i.e. Solid (solute) + Liquid
(solvent) Solution.
·
When the solid does not dissolve in the liquid, it is said to be insoluble.
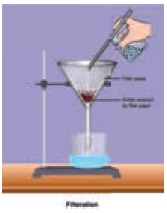
Separation of insoluble solids from liquids
Filtration
and Decantation: You are already
familiar with these methods. The illustrations given below will help you to
recall these important techniques.
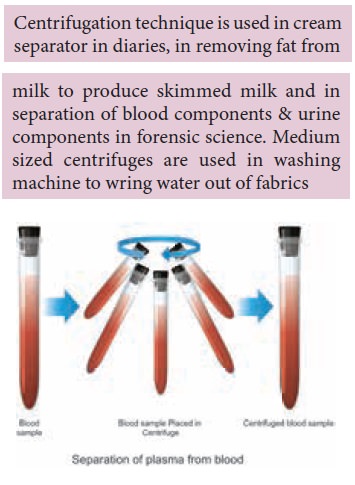
Centrifugation: is is
used to separate very ne and tiny
particles of solid which do not settle down easily in a liquid. The mixture
taken in a centrifuge tube is centrifuged (by rotation) in a centrifuging
machine, so that the solid gets deposited at the bottom of the tube and the
clear liquid (supernatant) is decanted. E.g. this is used to separate plasma
(the liquid) from blood.

Separation of soluble solids from liquids
Evaporation
and crystallisation: This is used
to separate the dissolved solute from the solution. The solution is heated
slowly so that the liquid (solvent) evaporates leaving behind the solid as
crystals. E.g. Separation of salt from sea water (by solar evaporation in
saltern).
Salterns in Tuticorin of Tamil Nadu
Simple
distillation: This method is
used to separate two liquids whose boiling points differ by more than 25 K.
Also by this method, brackish water can be distilled.

Procedure:
A
distillation flask is fixed with a
water condenser. A thermometer is introduced into the distillation flask
through a one-holed stopper. The bulb of the thermometer should be slightly
below the side tube.
The brackish water (sea water) to be distilled is
taken in the distillation flask and heated for boiling. The pure water vapour
passes through the inner tube of the condenser. The vapours on cooling condense
into pure water (distillate) and are collected in a receiver. The salt are left
behind in the flask as a residue.
3. Separation of liquid – liquid mixtures
a) Type I – Miscible liquids
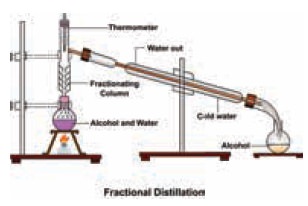
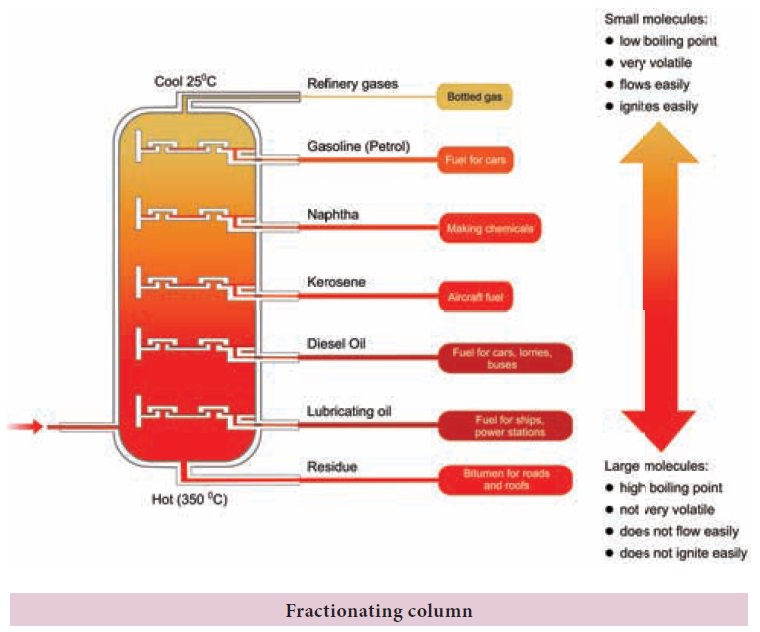
Fractional
distillation: To separate two or more miscible liquids which do not differ much in their boiling points
(difference in boiling points is less than 25 K) fractional distillation is
employed.
Example: Refining of petroleum product by fractional distillation.
Applications of fractional distillation
Fractional distillation is used in petrochemical
industry to obtain different fractions of petroleum, to separate the different
gases from air, to distil alcohols etc.
b) Type II: Immiscible liquids
Mixtures of two immiscible liquids are separated by
using a separating funnel.
Examples:
Mixture
of water and oil, Mixture of water
and kerosene.
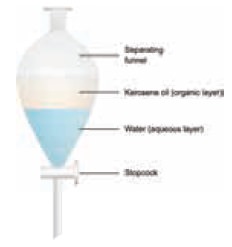
Two immiscible liquids can be separated by solvent
extraction method, which is also called as liquid – liquid extraction method.
is method works on the basis of difference in solubility of two immiscible
liquids in a suitable solvent. Solvent extraction method is used in soap,
pharmaceutical and petroleum industries.


Separation of mixture containing volatile and non-volatile solids
(i) Sublimation:
Certain
solid substances when heated change
directly from solid to gaseous state without attaining liquid state. The
vapours when cooled give back the solid substance. This process is known as
sublimation. Examples: (a) Iodine (violet vapours) (b) Camphor, (c) Ammonium
chloride etc.
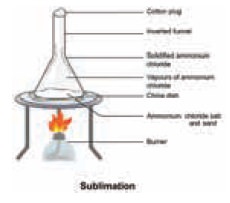
The powdered mixture of Ammonium chloride and sand
is taken in a china dish and covered with a perforated asbestos sheet. An
inverted funnel is placed over the asbestos sheet as shown in the gure. The open
end of the stem of the funnel is closed using cotton wool and the china dish is
carefully heated. The pure vapours of the volatile solid pass through the holes
in the asbestos sheet and condense on the inner sides of the funnel. The
non-volatile impurities remain in the china dish.
Separation of mixture containing volatile and non-volatile solids
Before we discuss the technique we will take a look
at two important terms that chromatography involves: Absorption and Adsorption.
Absorption
is the
process in which the substance is
dissolved throughout the bulk of another substance. For example a paper
(absorbent) soaks up or absorbs water.
Adsorption
is the
process in which particles of a
substance (it could be gas, liquid or dissolved solid) adhere to a surface of
another substance.
For example: charcoal adsorbs gases on its surface.
Charcoal is called the adsorbent and the gas is called the adsorbate.
Chromatography is a separation technique. It is
used to separate different components of a mixture based upon their different
solubilities in the same solvent.
There are several types of chromatography; based on
the above basic principles. It involves separation of mixtures by allowing the
constituents of the mixture to move between two phases namely
I. Mobile phase
II. Stationary phase
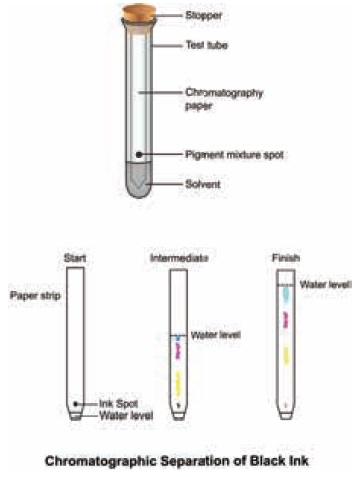
The simplest type is paper chromatography. Here,
the stationary phase is the chromatography paper and the mobile phase is the
solvent. For example, to separate the different-coloured dyes in a sample of
ink, a spot of the ink (e.g. black ink) is put on to a piece of chromatography
paper. is paper is then set in a suitable solvent as shown in Figure. The black
ink separates into its constituent dyes. As the solvent moves up the paper, the
dyes are carried with it and begin to separate. They separate because they have
different solubility in the solvent and are adsorbed to different extents by
the chromatography paper. The chromatogram shows that the black ink contains
three dyes.
We can also draw important inferences from a
numerical measurement called Rf (Retention factor) values using the obtained
chromatograms. Rf value is defined as the ratio of the distance travelled by
the solute spots to the distance travelled by the solvent.

Applications
Chromatography is used extensively in medical
research and forensic science laboratories to separate a variety of mixtures.
For example, protein samples are separated by electrophoresis in medical
research laboratories.
Related Topics